

(ii) Molecular Orbitals 1 - sp3 hybridisation
(iii) Molecular Orbitals 1 - sp2 hybridisation
(iv) Colour in Organic Molecules
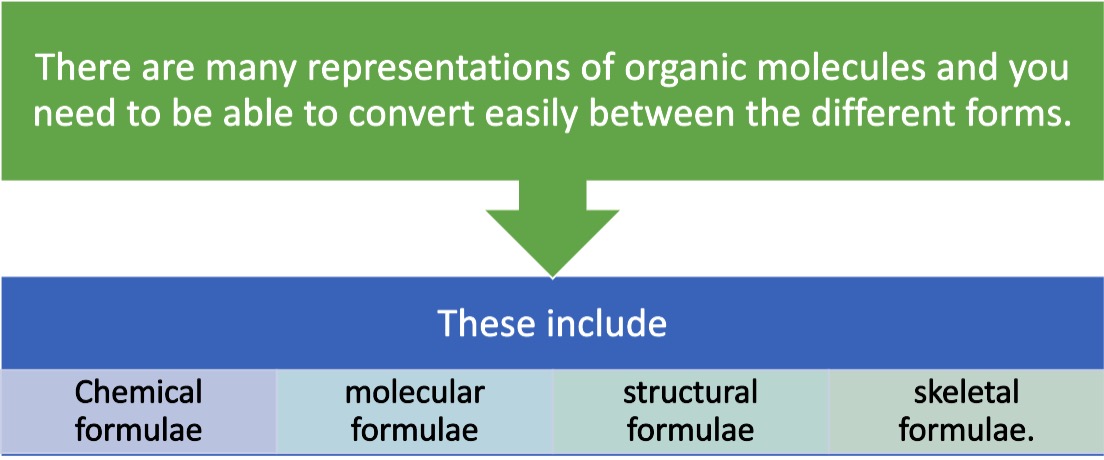
The molecular formula tells us the ratio of elements within an organic molecule.
e.g. of butane is C4H10 telling us we have a molecule with the elements carbon and hydrogen in the ration 4:10 respectively.
The table below shows some molecular formulae for a few selected hydrocarbon molecules.
| Name | Molecular formula |
|---|---|
| ethane | C2H6 |
| propane | C3H8 |
| butene | C4H8 |
| pentene | C5H10 |
| cyclobutane | C4H8 |
| cyclohexane | C6H12 |
For molecules with functional groups including hydroxyl (OH), amine (NH2), carboxyl (COOH) and carbonyl (CO) these are shown as part of the molecular formula of the molecule.
| Name | Molecular formula |
|---|---|
| ethanol | C2H5OH |
| ethanoic acid | CH3COOH |
| ethylamine | C2H5NH2 |
| ethanal | CH3CO |
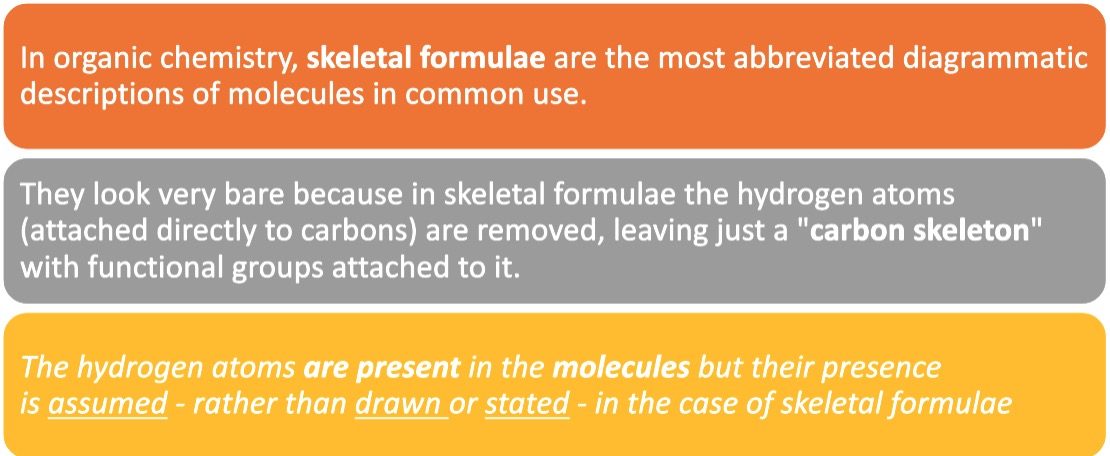
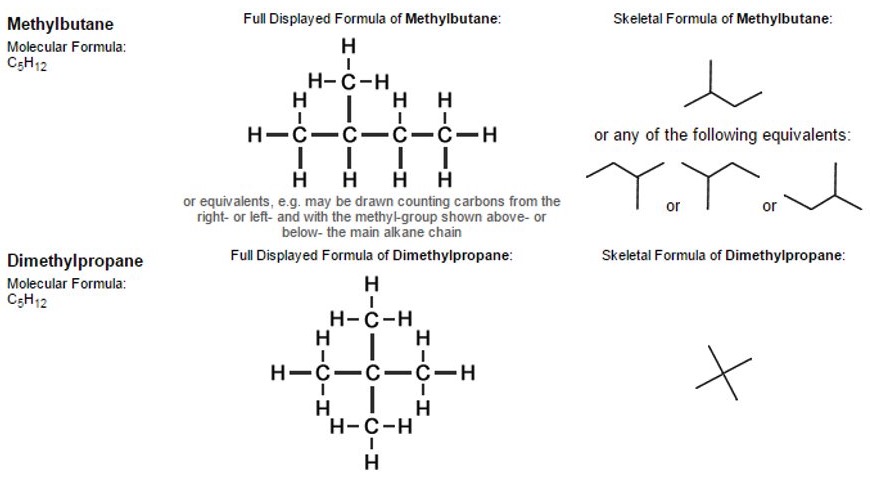
The simplest examples of skeletal formulae are those for linear alkanes.
So, a "chain link" of two carbon atoms linked together by a single covalent bond (together with the hydrogen atoms attached to them) is represented by a single line when drawn in the form of a skeletal formula.
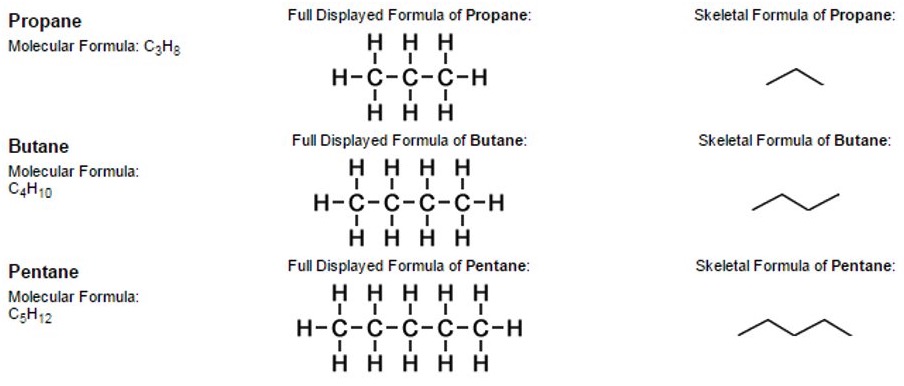
Notice that it is not necessary to write the symbols "C" or "H" for atoms that form part of simple linear chains, as opposed to other structures such as functional groups attached to alkane-chains.
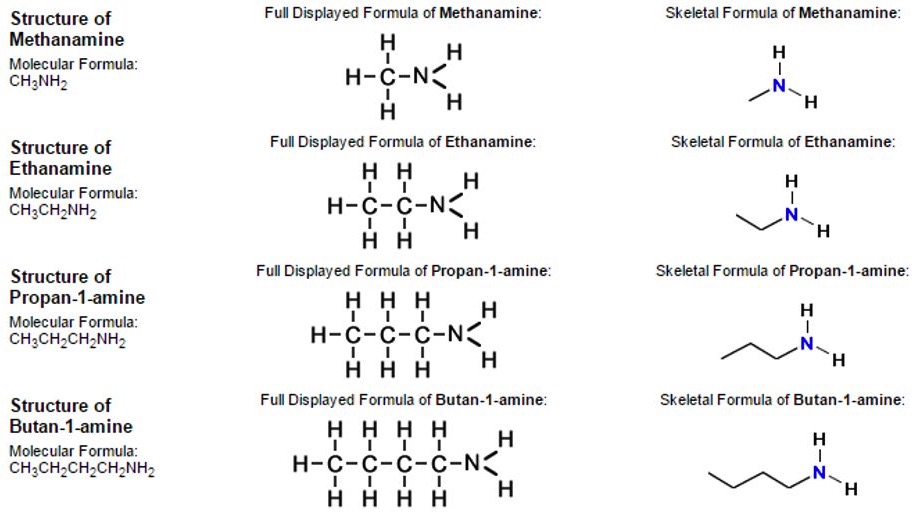
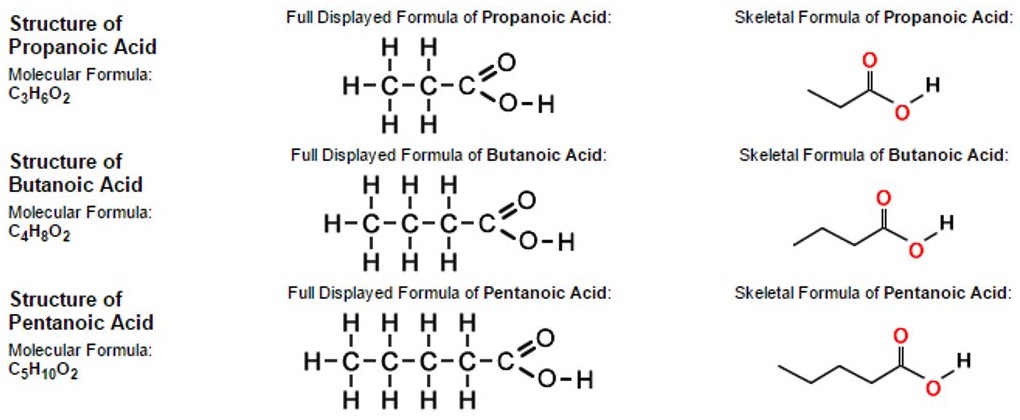

Organic compounds are organised into groups according to similarities and differences in their structure.
Groups of atoms within a molecule give the molecule specific characteristic properties.
These groups of atoms are known as FUNCTIONAL GROUPS.
Hydrocarbons - Compounds containing only carbon and hydrogen.
Subdivisions
In the ground state, carbon has the electron arrangement 1s2 2s2 2p2.
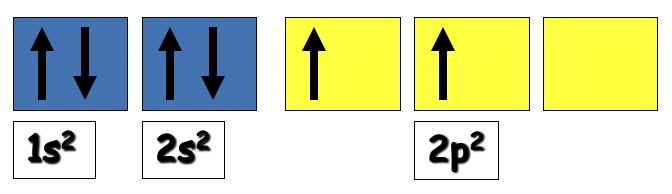
The 2p2 electrons occupy 2 of the 3 p orbital (Hund's Rule)
This suggests that there are only two electrons available to form covalent bonds. However, carbon forms four bonds.
This is explained by the idea of Hybrid orbitals.
Hybrid orbitals are the result of 'mixing' orbitals to produce a new set of orbitals.
Mathematical basis for this doing this is based on the Schrodinger wave equations - not needed at this level!
Spectroscopic measurements show that all four bonds in methane are identical.
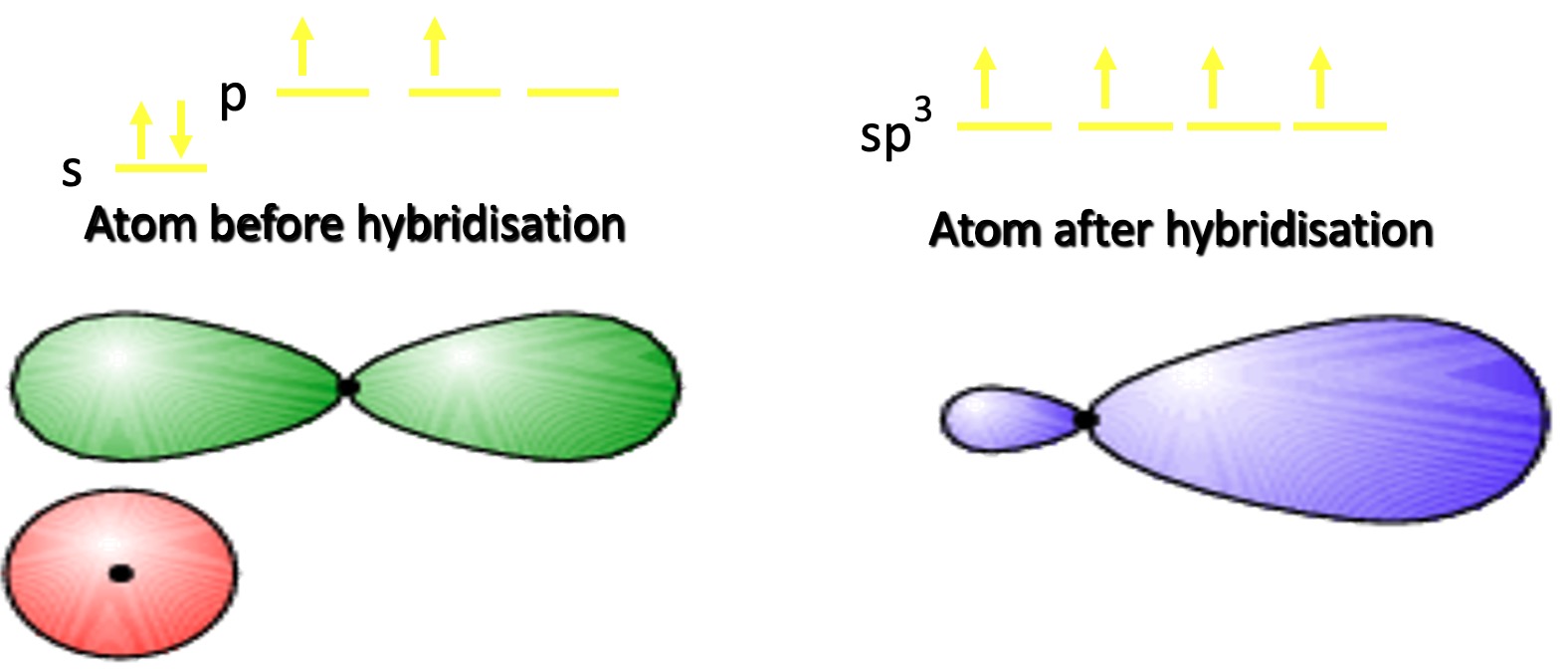
One of the 2s electrons is promoted to the third p orbital.
This produces 4 Hybrid orbitals of equal energy containing single electrons. This is known as sp3 hybridisation.
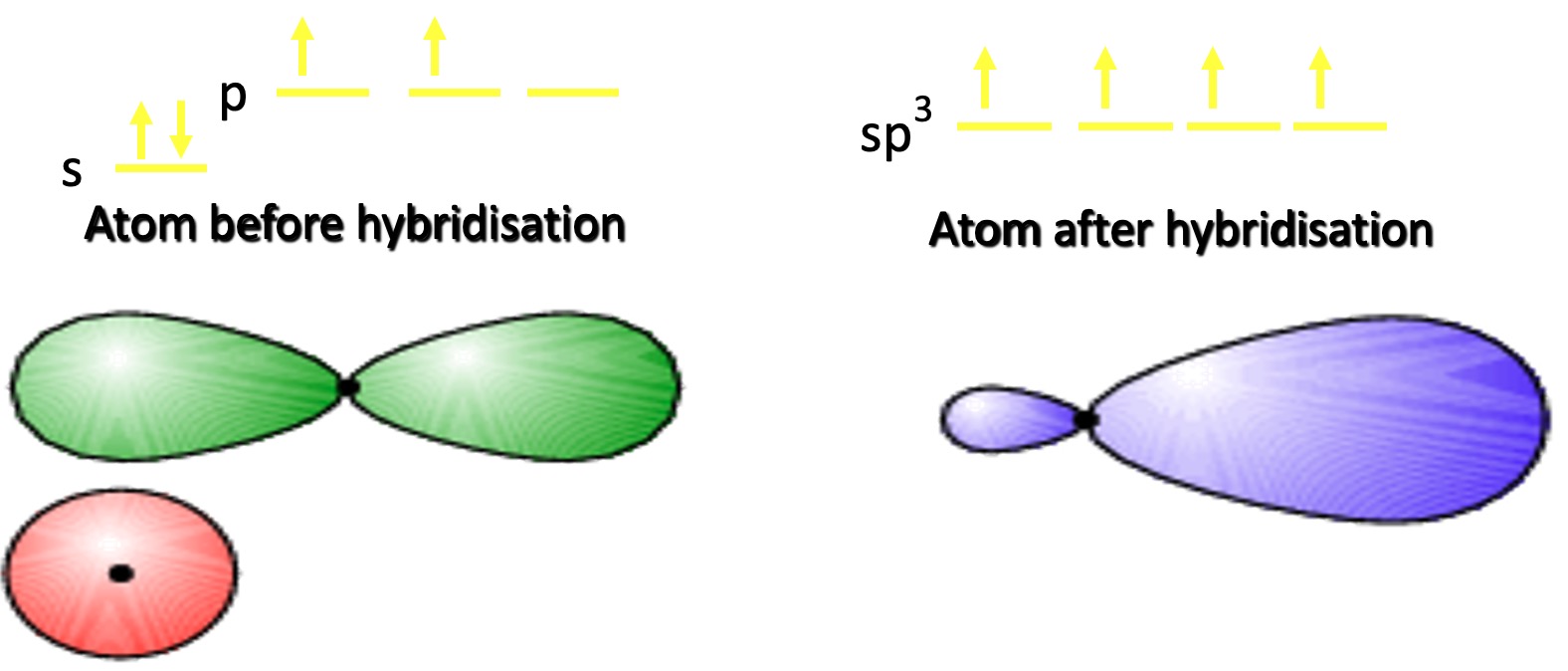
Compare the shape of the p orbital for carbon with that of the sp3 orbital.
The hybrid orbital is more directional in shape → better overlap when forming bonds + stronger bonds + forms 4 bonds.
For simplicity, the smaller lobe is often omitted from diagrams.
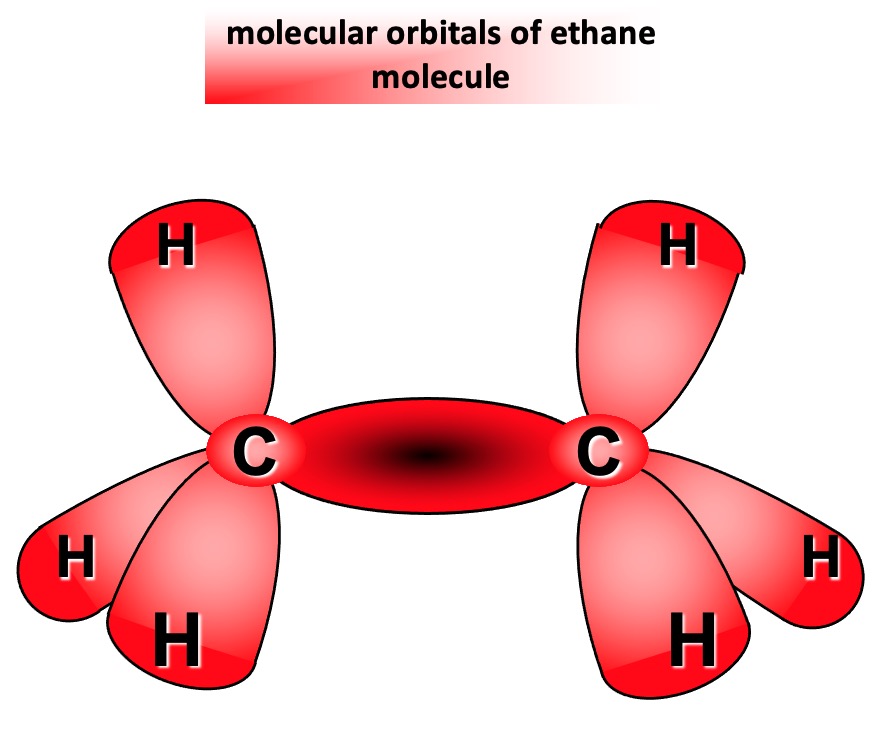
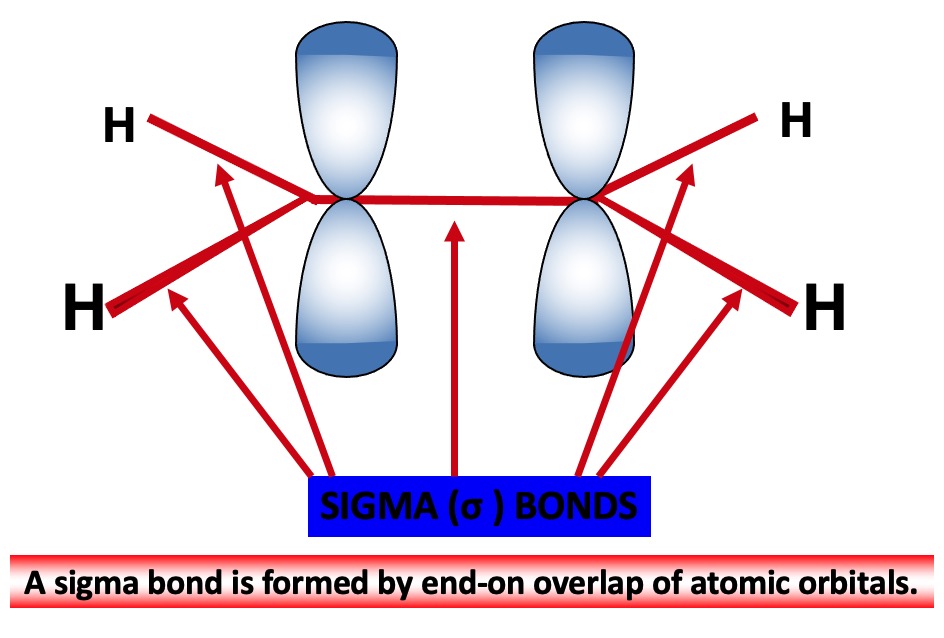
Overlap of a half-filled sp3 hybrid orbital with a half-filled 1s orbital of a hydrogen atom forms a new molecular orbital The shared pair of electrons are now under the influence of both nuclei.
The new molecular orbital lies along the axis joining the two nuclei and is known as a SIGMA (σ) BOND. A sigma bond is formed by end-on overlap of atomic orbitals.
C = C double bonds are formed by sp2 hybridisation.
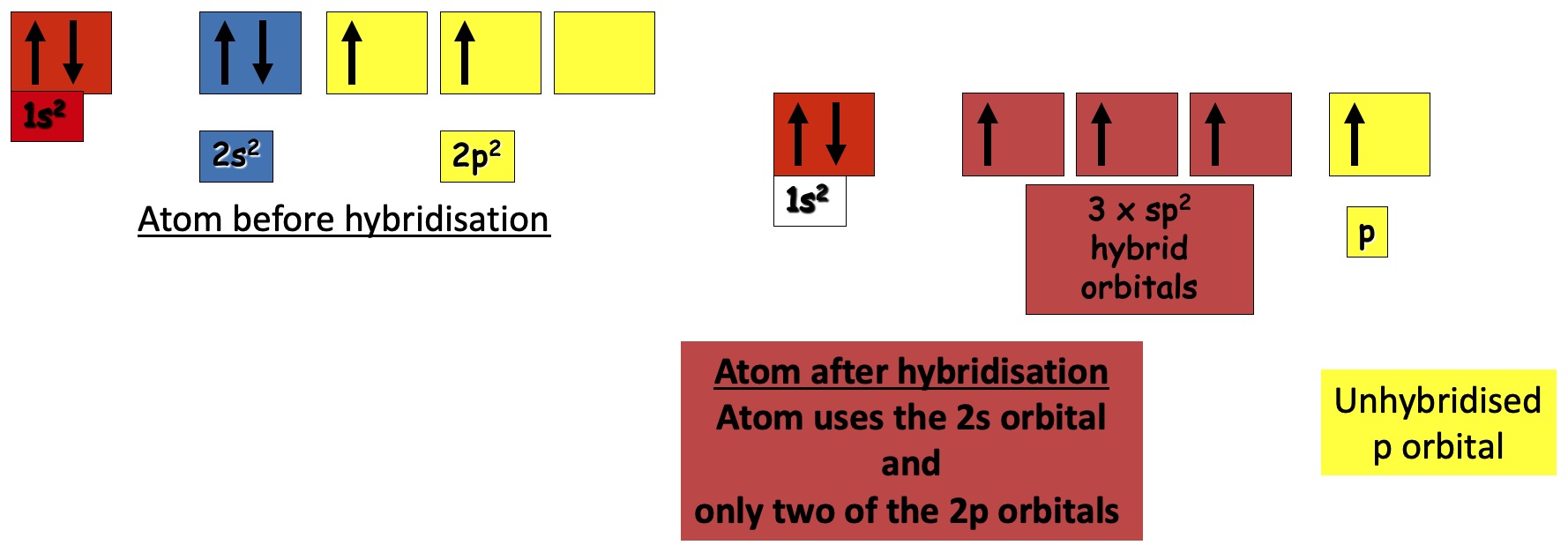
On mathematically mixing these, three identical hybrid orbitals are obtained.
These lie in the same plane at 120o to each other and at 90o to the remaining p orbital.
This is called sp2 hybridisation and the new orbitals are called sp2 hybrid orbitals.
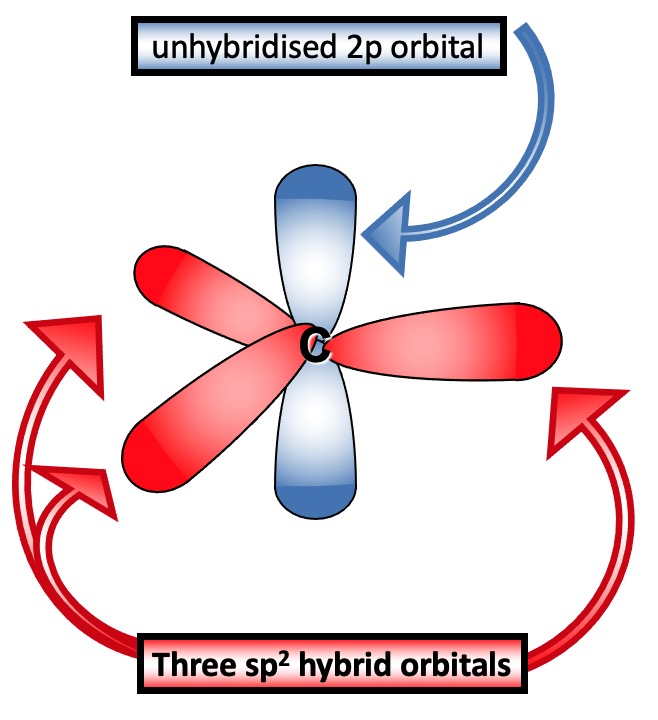
Unhybridised p orbitals are close enough to overlap above and below the plane of the sp2 bonds.
This produces a new molecular orbital between the 2 carbon atoms with lobes above and below the molecular plane.
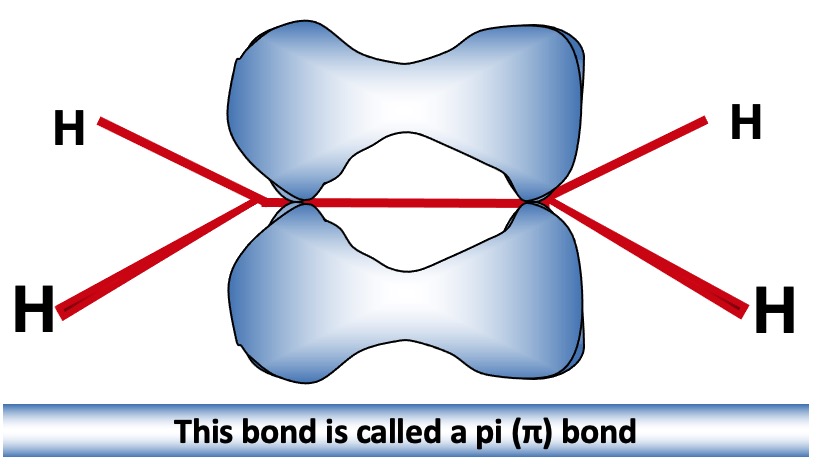
This extra bond pulls the carbon atoms closer together - bond length shortens
Sideways overlap produces a weaker bond than the end-on overlap → C=C isn't 2 x as strong as C-C
π overlap is more effective when the p orbitals are parallel. Any other position and the overlap is reduced and the bond will eventually be broken → resistance to rotation.
Molecular orbital theory can be used to explain why organic molecules are colourless or coloured. Electrons fill bonding molecular orbitals, leaving higher energy antibonding orbitals unfilled. The highest bonding molecular orbital containing electrons is called the highest occupied molecular orbital (HOMO). The lowest antibonding molecular orbital is called the lowest unoccupied molecular orbital (LUMO).
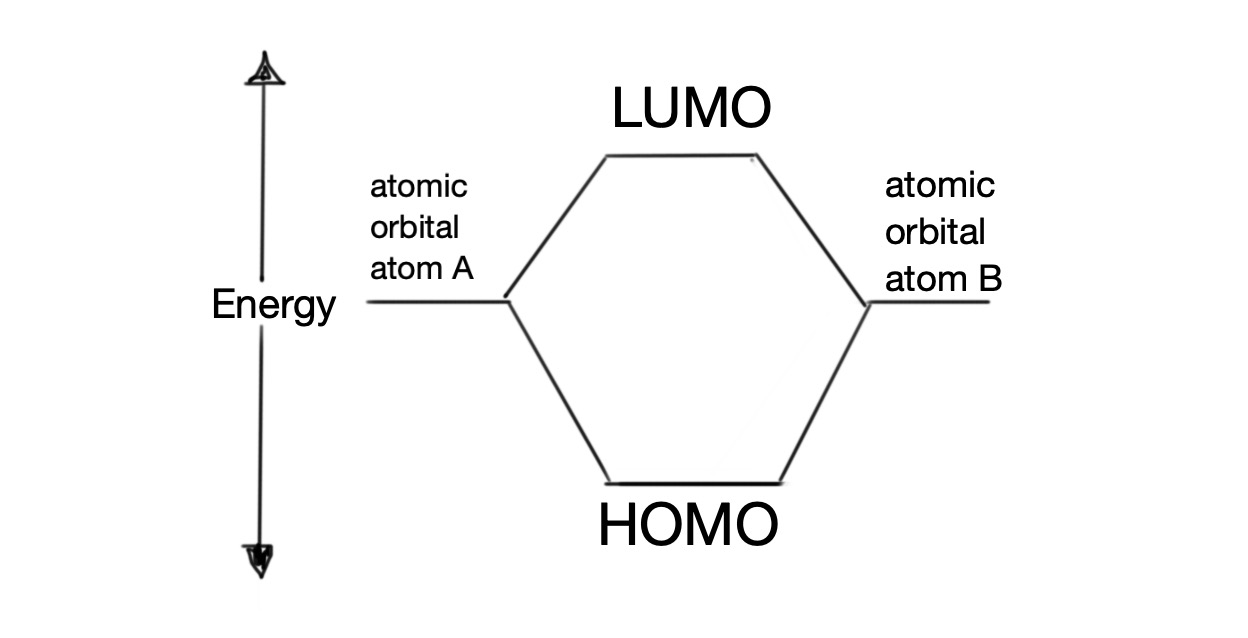
Absorption of electromagnetic energy can cause electrons to be promoted from HOMO to LUMO.
Most organic molecules appear colourless because the energy difference between HOMO and LUMO is relatively large. This results in absorption of light from the ultraviolet region of the spectrum.
Some organic molecules contain chromophores. A chromophore is a group of atoms within a molecule that is responsible for absorption of light in the visible region of the spectrum. Light can be absorbed when electrons in a chromophore are promoted from the HOMO to the LUMO.
Chromophores exist in molecules containing a conjugated system - a system of adjacent unhybridised p orbitals that overlap side-on to form a molecular orbital across a number of carbon atoms. Electrons within this conjugated system are delocalised. Molecules with alternating single and double bonds, and aromatic molecules have conjugated systems.
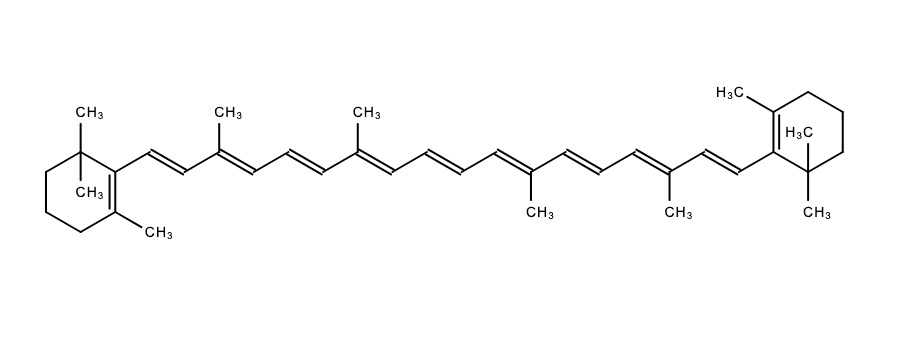
The more atoms in the conjugated system the smaller the energy gap between HOMO and LUMO. A lower frequency of light (longer wavelength, lower energy) is absorbed by the compound. When the wavelength of light absorbed is in the visible region, the compound will exhibit the complementary colour.