

(i) Synthesis 1: Bond Fission, Electrophiles and Nucleophiles
(iii) Synthesis 3: Mechanisms of Nucleophilic Substitution Reactions
(iv) Synthesis 4: Alcohols and Ethers
(vi) Synthesis 6: Mechanisms of Electrophilic Addition Reactions
(viii) Synthesis 8: Carboxylic Acids
In this section you will learn the synthetic routes used to prepare organic molecules. Some of the reactions will be familiar from Higher and National 5. You will need to know the reaction conditions, reagents as well as some of the reaction mechanisms for the reactions below.
Bond fission relates to the breaking of bonds. There are two types, homolytic fission and hetrolytic fission
When a non-polar covalent bond is broken, one electron from the σ-molecular orbital moves back to each of the atoms from which the original bond was formed. This is what happens in the initiation step of the free radical chain reaction. This can be seen in the diagram below:
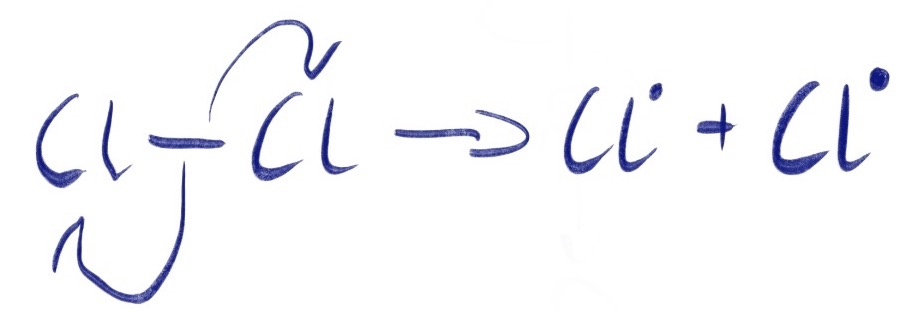
The 'half headed' curly arrows show where each electron originates.
Heterolytic fission happens when polar covalent bonds are broken. On this occasion, both electrons are transferred to the more electronegative atom as in the example below:

The double headed curly arrow indicates the transfer of both bonding electrons to atom 'B'. The head shows the destination of the electrons and the tail shows the origin.
If 'B-' were to go on to react with an atom 'C+', the head of a double headed curly arrow would would point to the space in between 'B' and 'C' to indicate where the double bond would be formed.
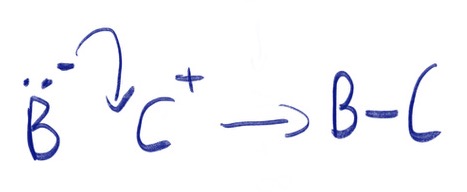
In the example above, the negatively charged atom 'B-' donate a pair of electrons to the positively charged atom 'C+'. Atom 'B-' is said to be acting as a nucleophile as it is donating electrons. Examples include Br-, OH- and NH3. Atom 'C+' is said to be acting as an electrophile as it is accepting electrons. Examples include H+, NO2+ and SO3.
Which of the following has nucleophilic properties?
Which of the following does not occur in the reaction between methane and chlorine?
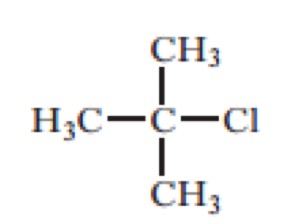
Which of the following would be the most likely products of the heterolytic bond fission of the above compound?
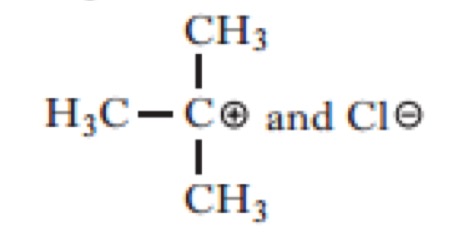
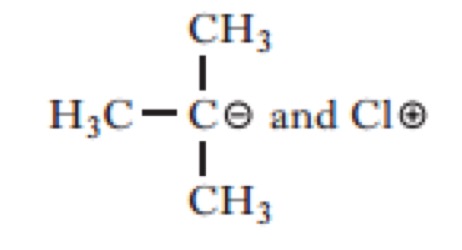
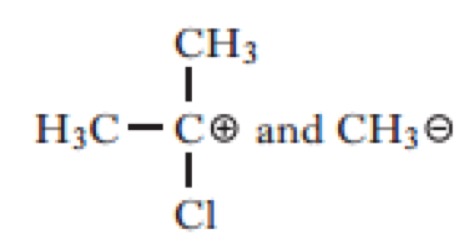
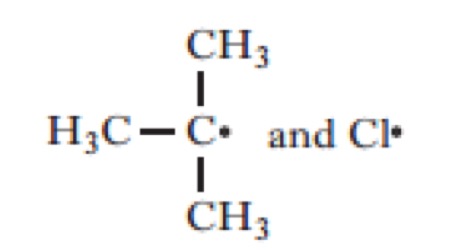
Click the reaction button bellow to display a chemical reaction.
Click show answer to reveal the reaction type
Haloalkanes are alakanes that have a halogen substituted in place of a hydrogen. They are named in a similar way to alkanes, the location of the alkyl chain is given the lowest possible number.
As with alcohols, there are three types of monohalide primary, secondary and tertiary.
Haloalkanes can be synthesised using the free radical chain reaction. You learned this in Higher Chemistry.
The video below is a reminder of the free radical chain reaction and the reaction mechanism involved.
As halogens are more electronegative than carbon, monohaloalkanes have a polar carbon to halogen bond. This causes the carbon to vulnerable to nucleophilic attack. The incoming nucleophile donates a pair of electrons to the carbon atom, forming a new covalent bond. This causes the halogen to be thrown out or substituted by the incoming nucleophile.
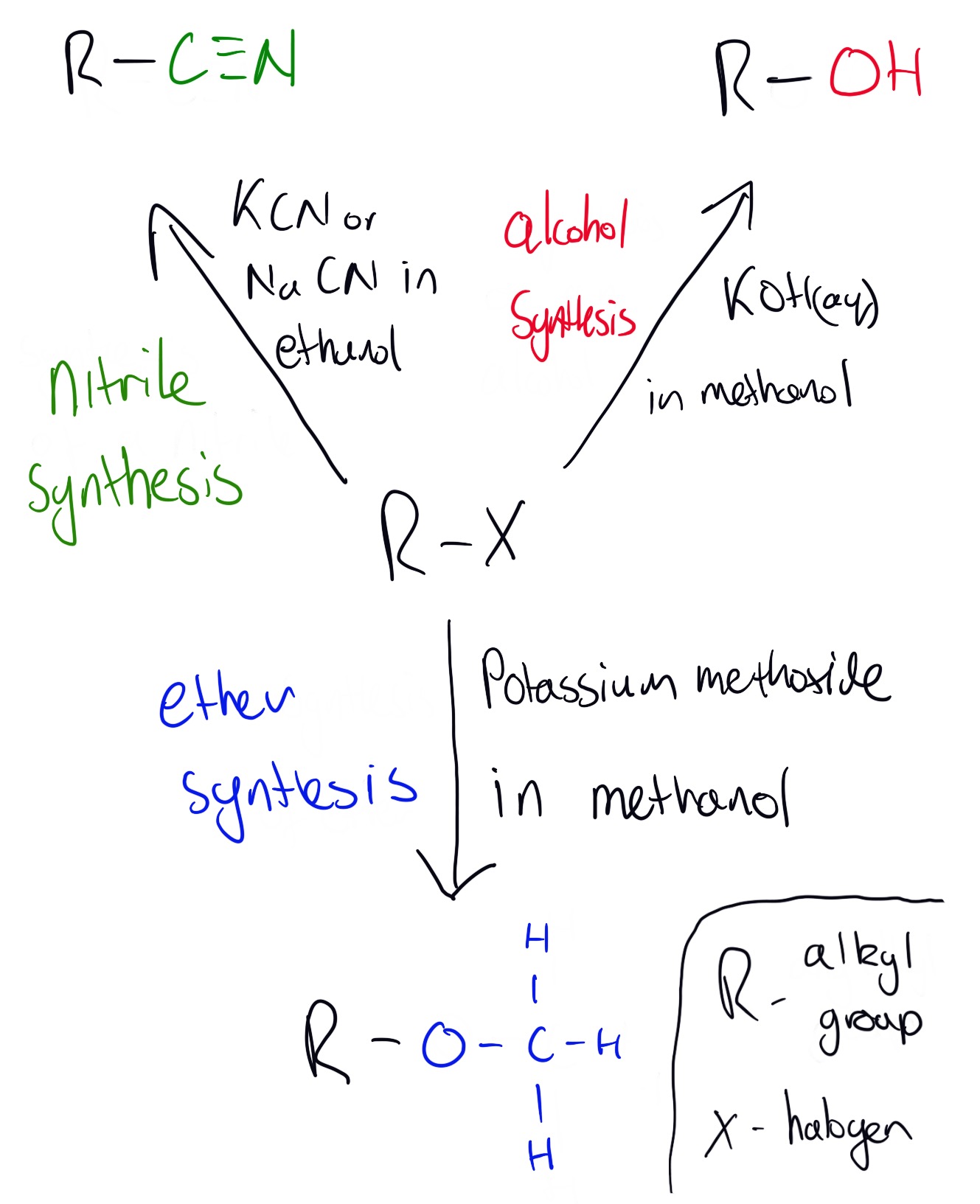
Monohaloalkanes can undergo the following nucleophilic substitution reactions shown below.
Monohaloalkanes can undergo elimination reactions when they are heated under reflux with ethanolic potassium (or sodium) hydroxide.
In the formation of the carbon to carbon double bond, the halogen atom and an H atom on an adjacent carbon atom is removed from the haloalkane and not replaced. This reaction is reffered to as a base - induced elimination reaction.
The elimination of hydrogen halides from monohloalkanes can result in the formation of alkenes.
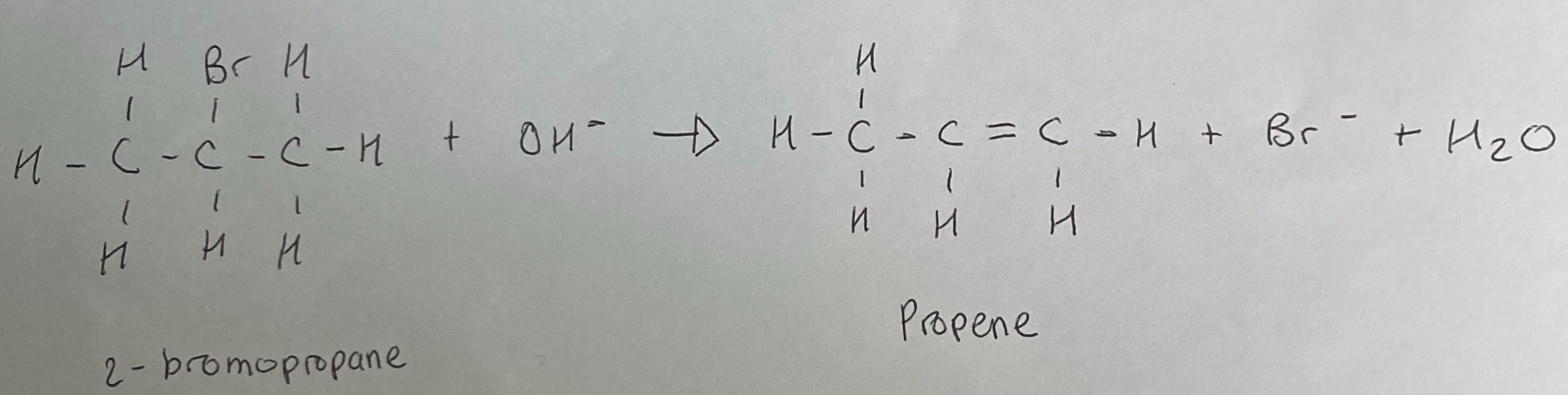
1. What are haloalkanes (alkyl halides)
Substituted alkanes in which one or more of the hydrogen atoms is replaced with a halogen atom.
2. How many halogens do monohaloalkanes contain?
1
3. What does the classification primary, secondary and tertiary mean with respect to haloalkanes?
This tells you the number of alkyl branches bonded to the carbon atom that is attached to the halogen.
4. How is the presence of a halogen atom denoted?
By using the start of the halogens name as a prefix e.g. fluoro-, chloro etc
5. What is formed when haloalkanes undergo an elimination reaction using a string base such as sodium or potassium hydroxide?
Alkenes
6. Name three products products of nucleophilic substitution reactions with monohaloalkanes and name the reagents required?
1. alcohols using aqueous alkalis as reagents 2. nitriles using ethanolic cyanide. Product can be hydrolised to form a carboxylic acid. 3. ethers using alcohlic alkoxides
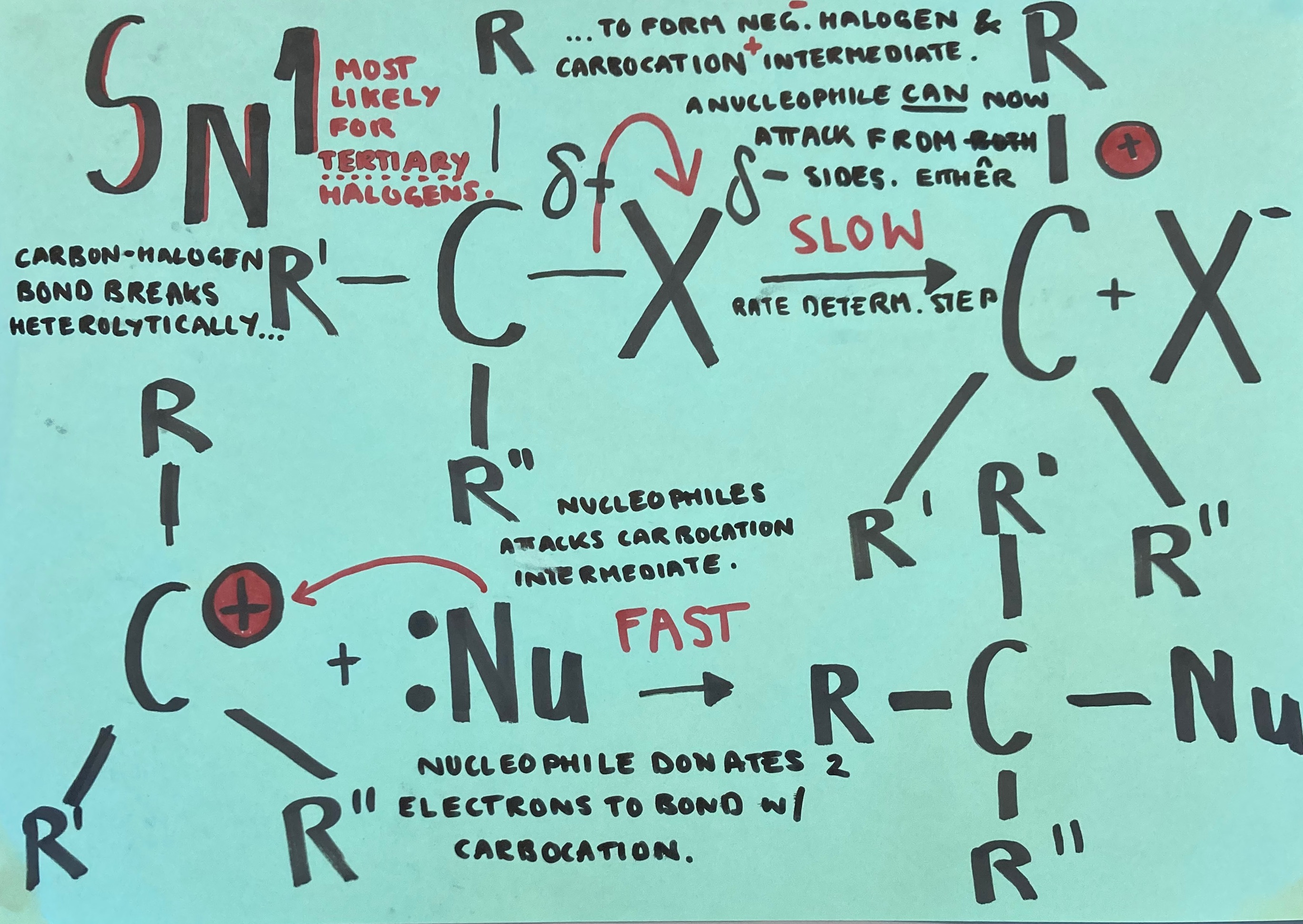
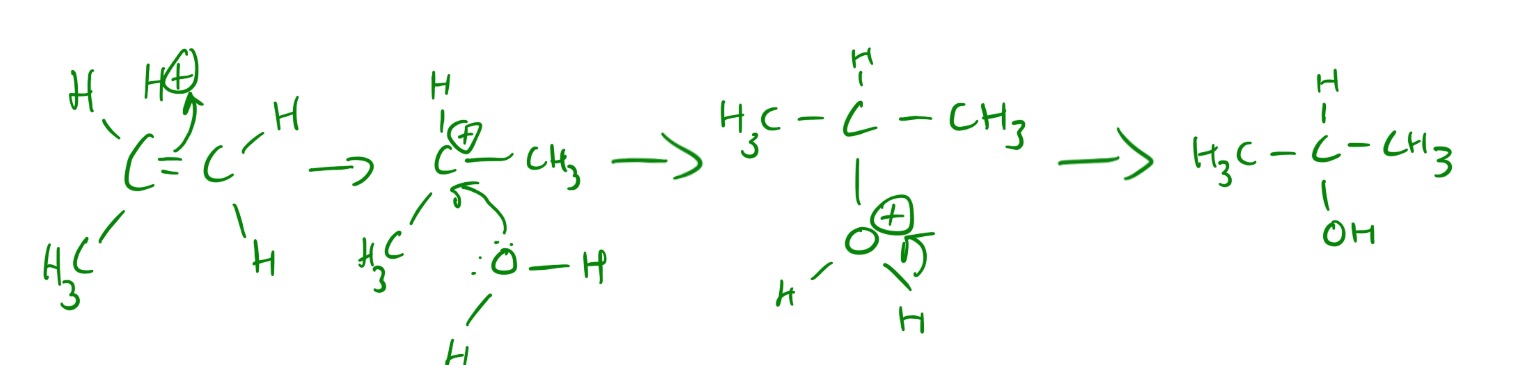
The Sn1 mechanism is explained in the picture below.
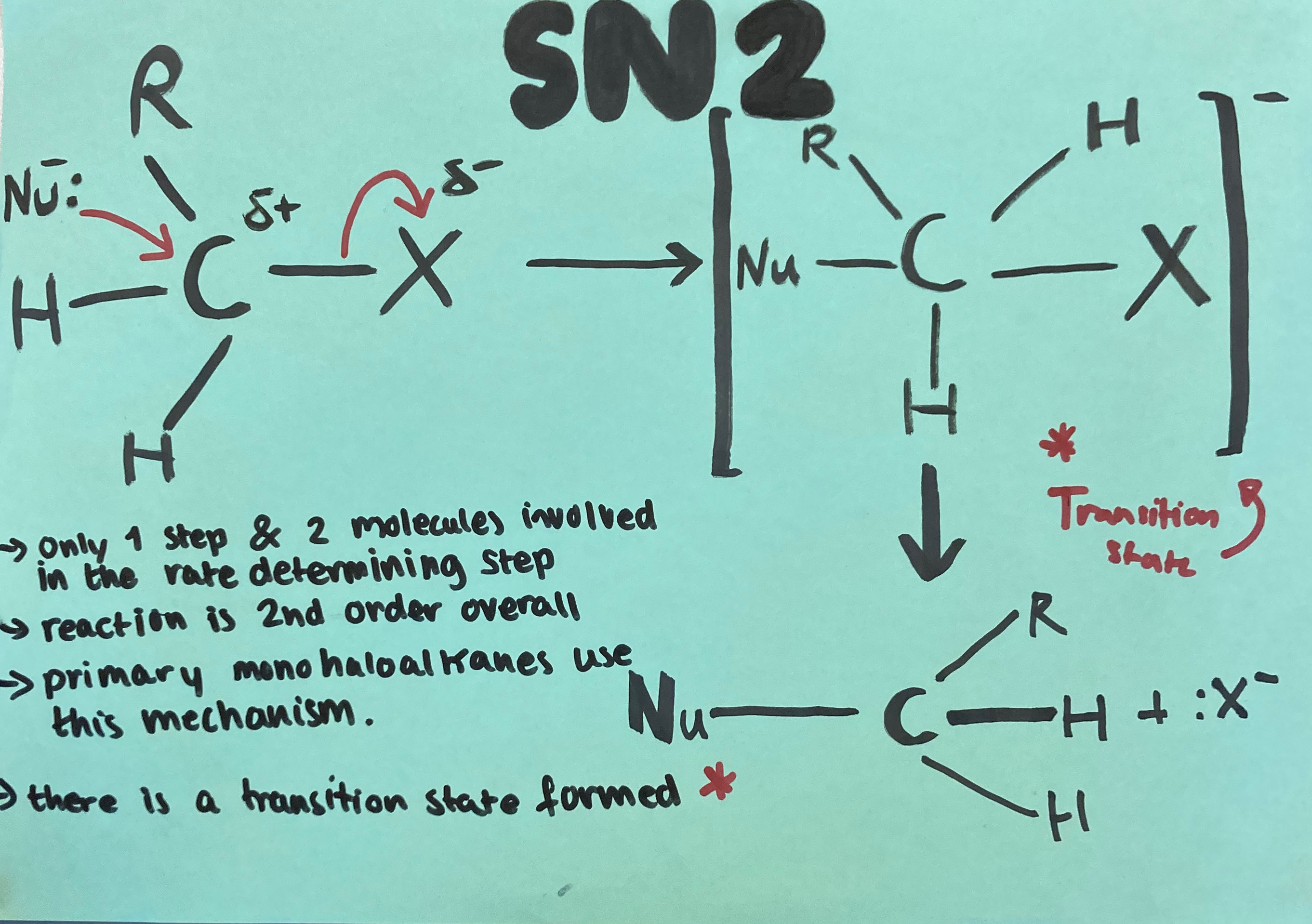
The Sn2 mechanism is explained in the picture below.
Explanations of Sn1 and Sn2 reaction mechanisms can be seen below.
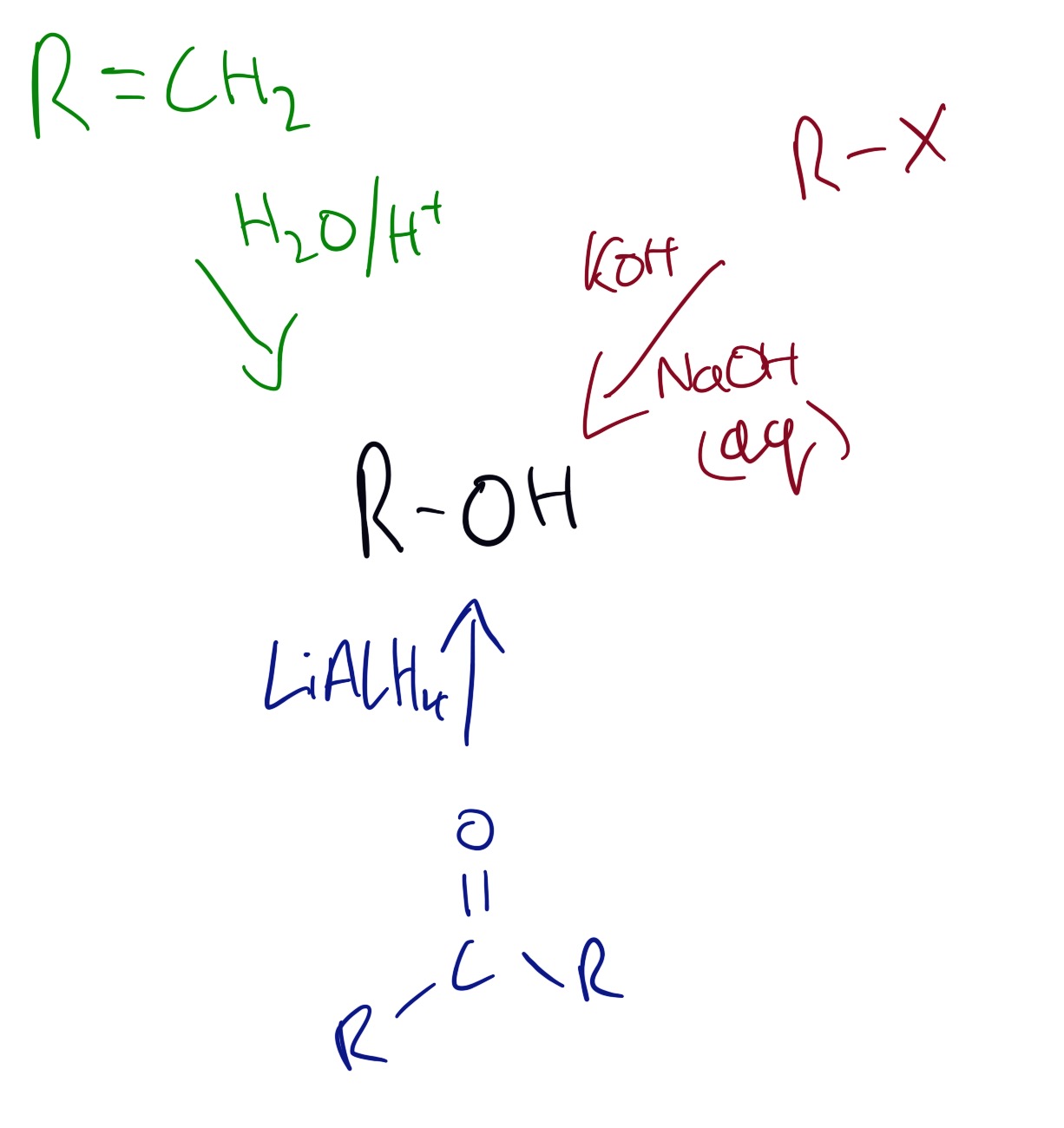
Alcohols can be produced by the reactions shown in the diagram below:
Reactions of alcohols can be seen in the diagram below.
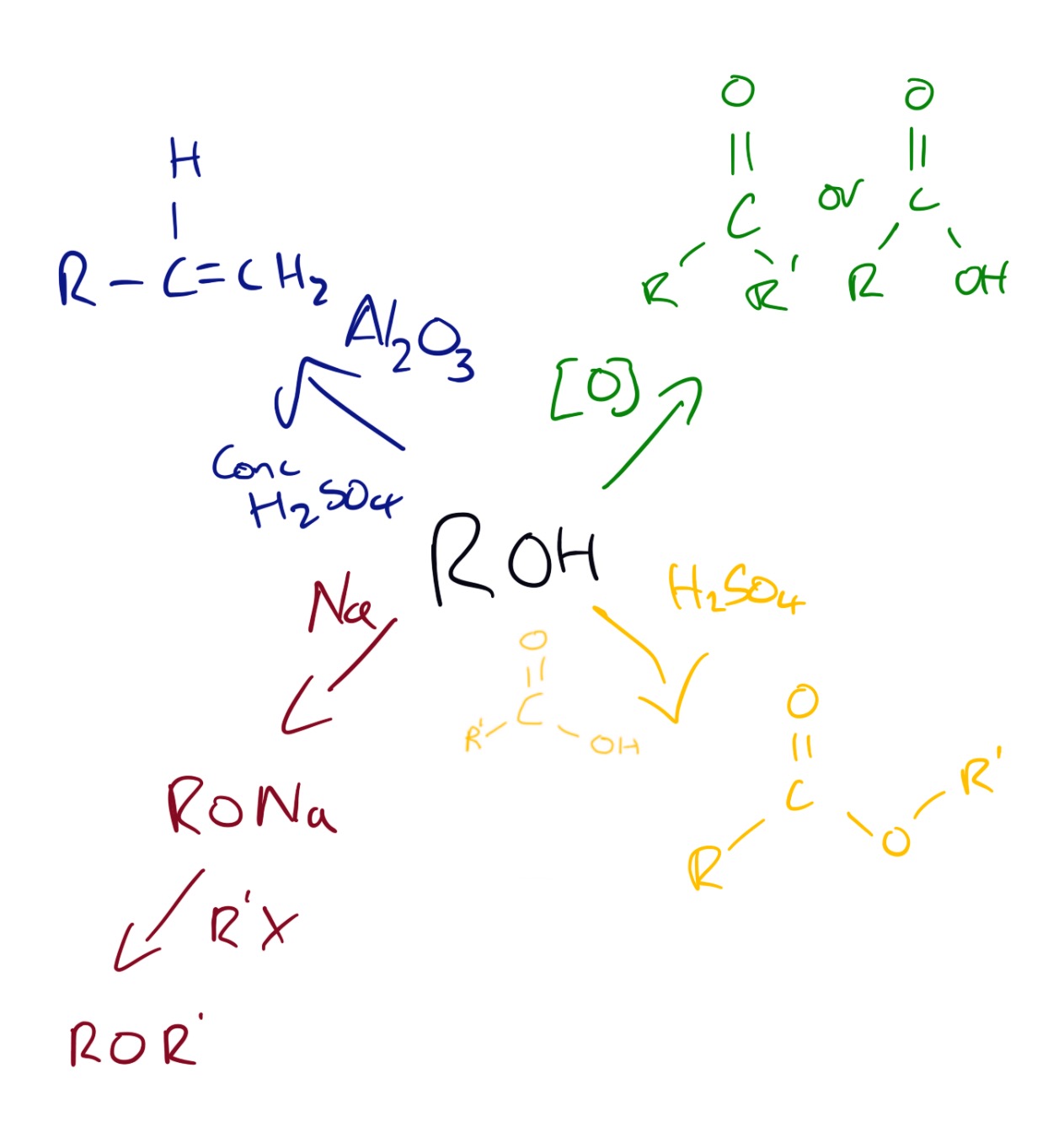
Explanations of the reactions above can be seen in the videos below.
The name of the ether below can be found by following the steps below:

Ethers are prepared in two steps. First an alkali metal is reacted with an alcohol to form a metal alkoxide. The metal alkoxide is then reacted with a monoholoalkane in a substitution reaction. This is shown below.
The video below explains how ethers are named, their properties and how they are produced.
Click the reaction button bellow to display a chemical reaction.
Click show answer to reveal the reaction type
Along with catalytic cracking there are two ways of producing alkenes. These can be seen in the picture below.
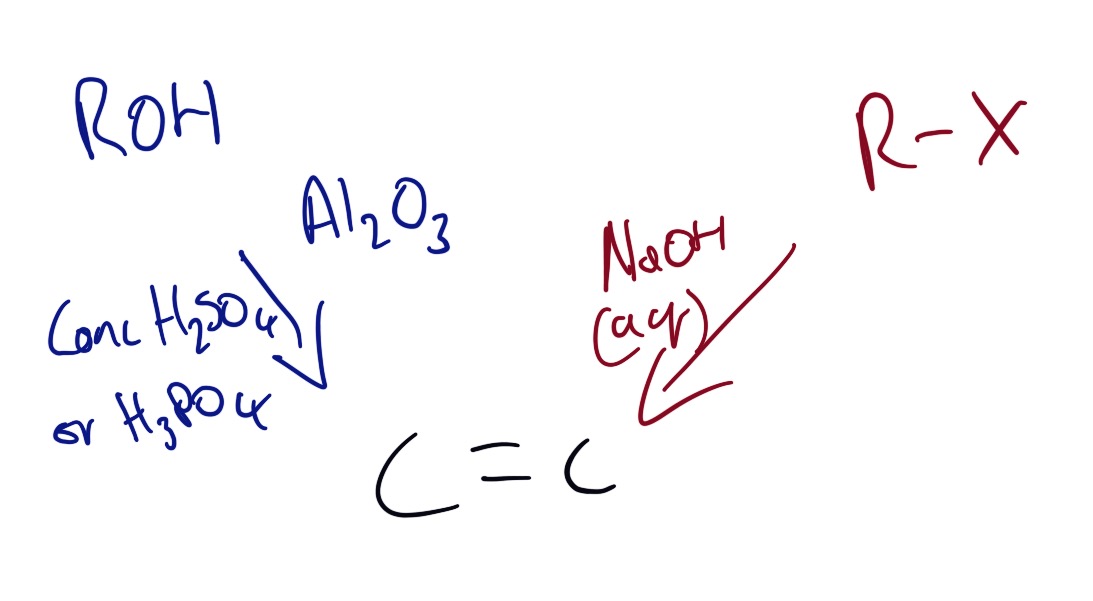
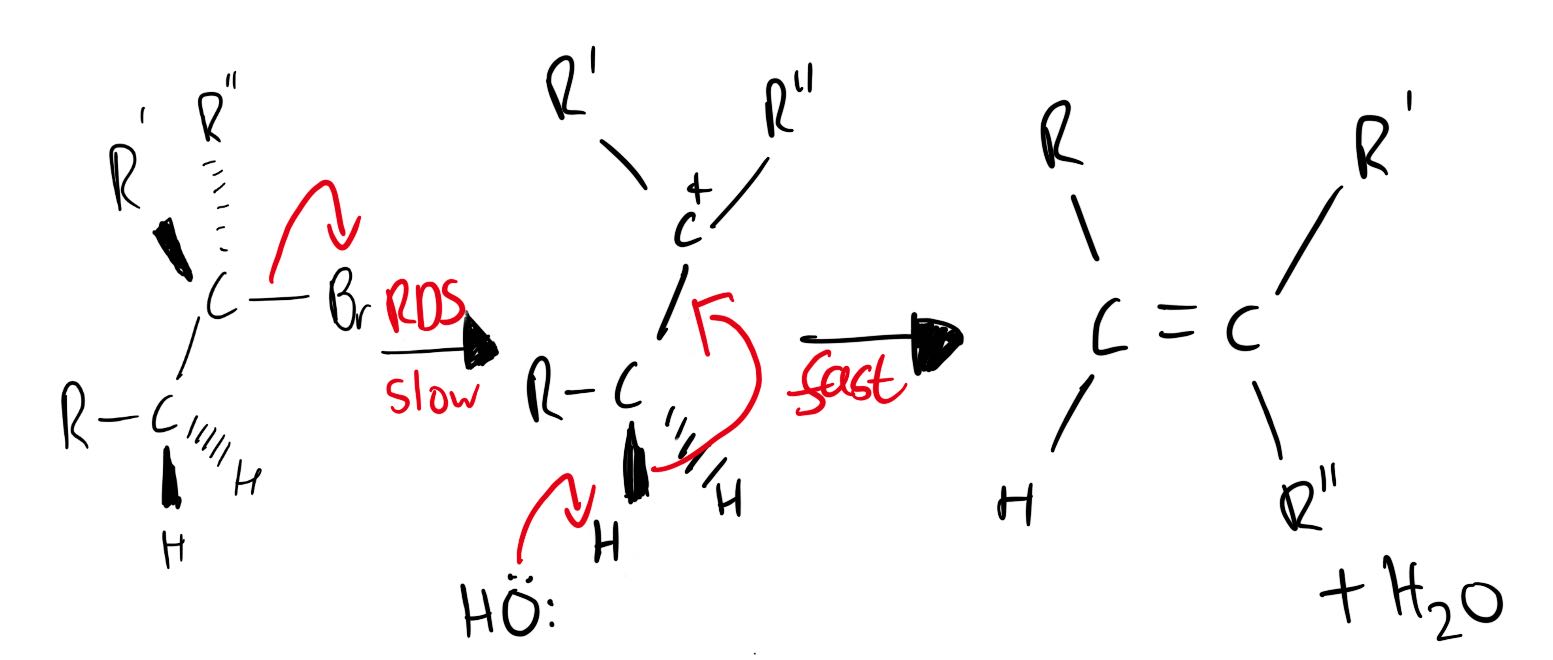
Mechanism is not required but has been included for interest.
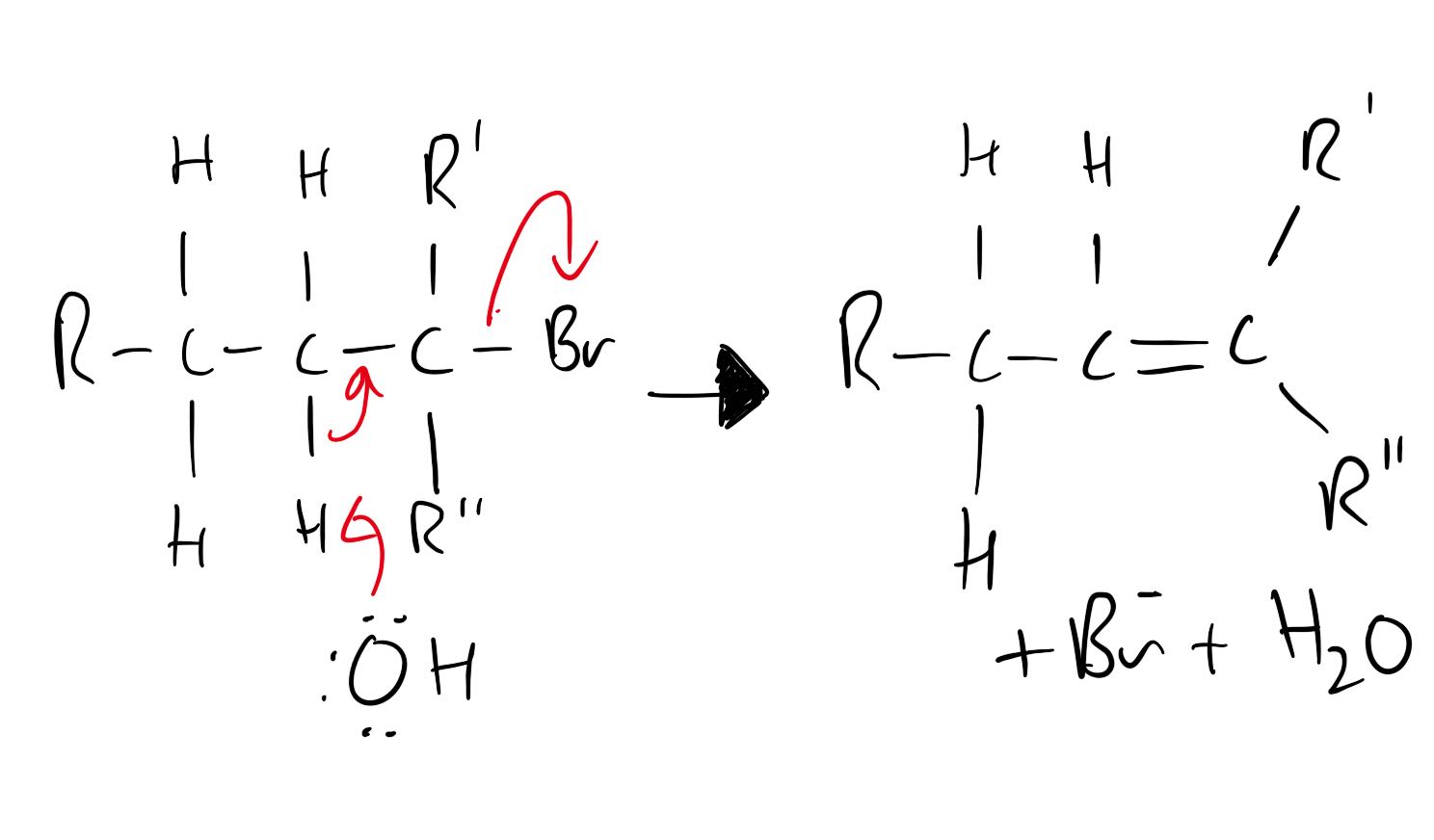
Mechanism is not required but has been included for interest.
Reactions of the alkenes can be seen below.
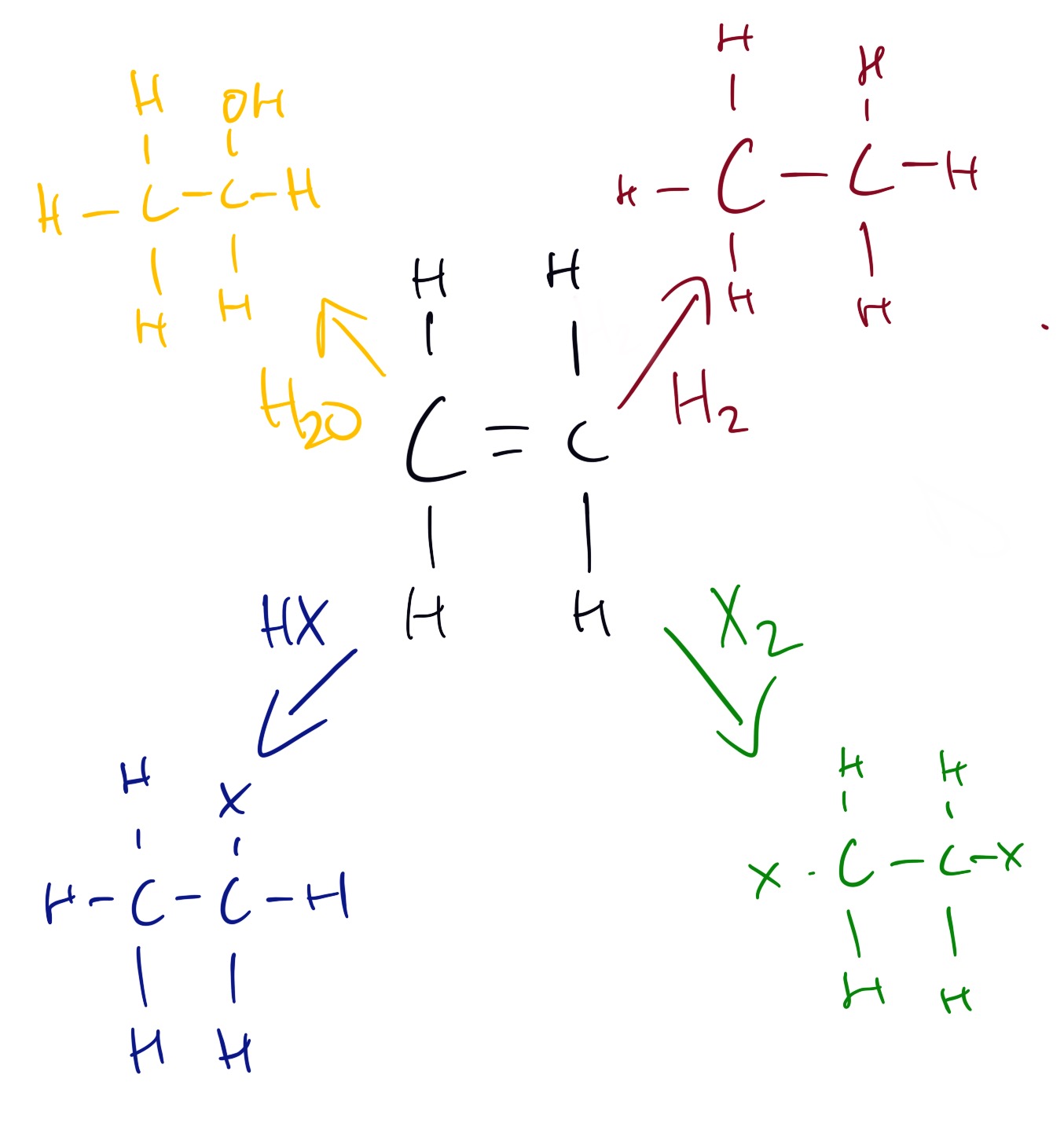
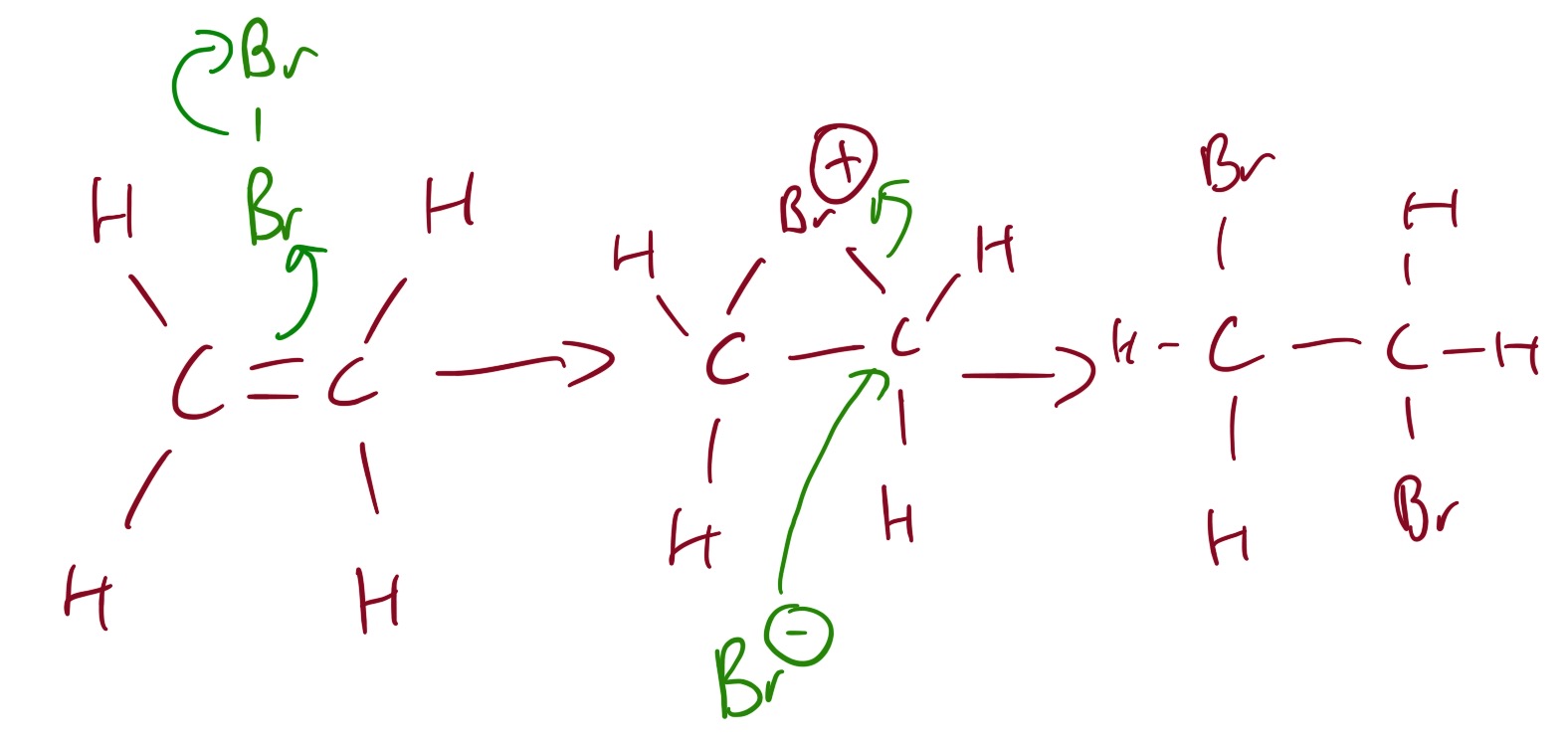
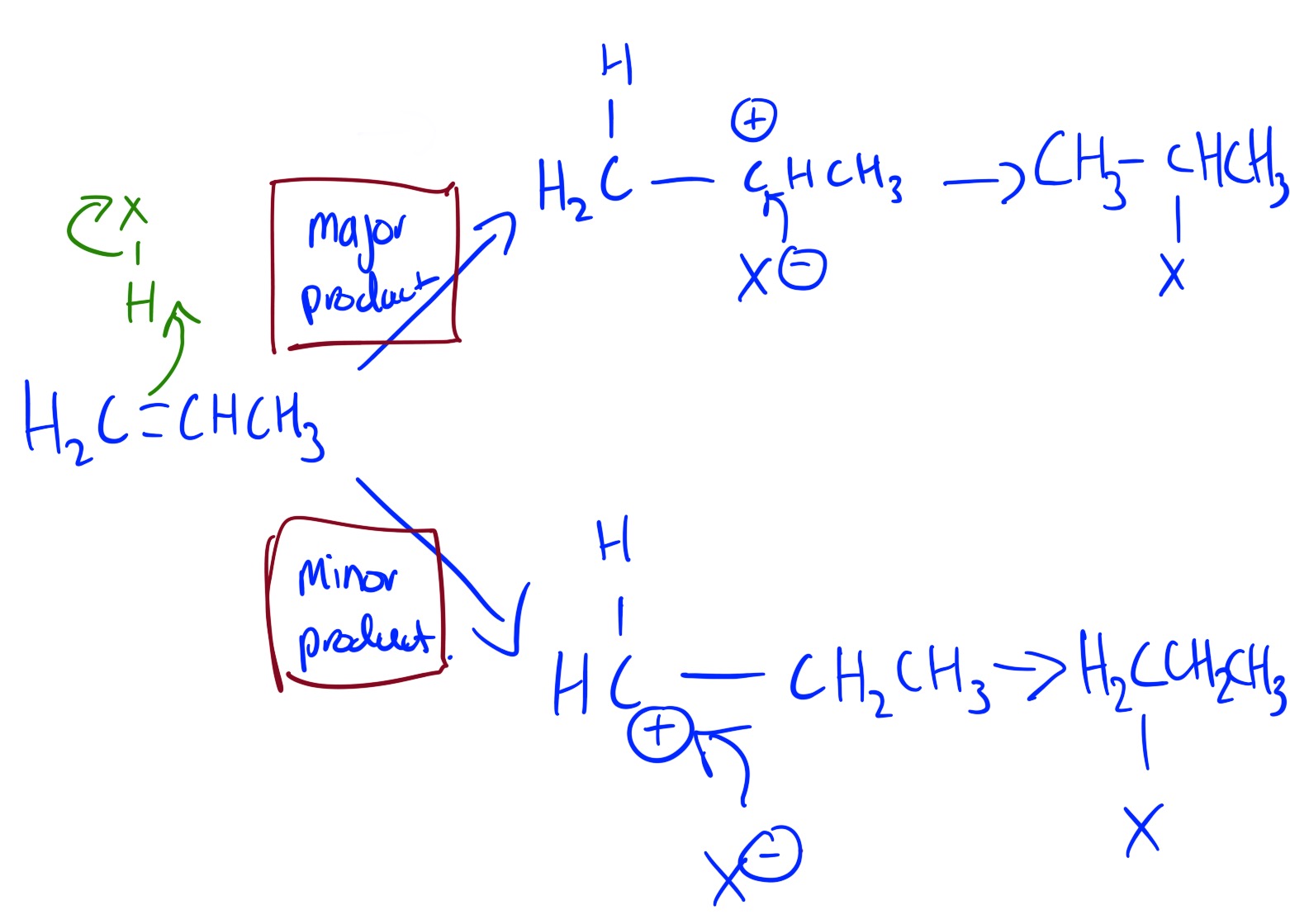
The major product is 2-halopropane. This is because it forms a more stable carbocation intermediate. This is an example of Markovnikiv's rule.
The video below explains how alkenes are produced, their reactions and reaction mechanisms.
The reactions of the amines are shown below.
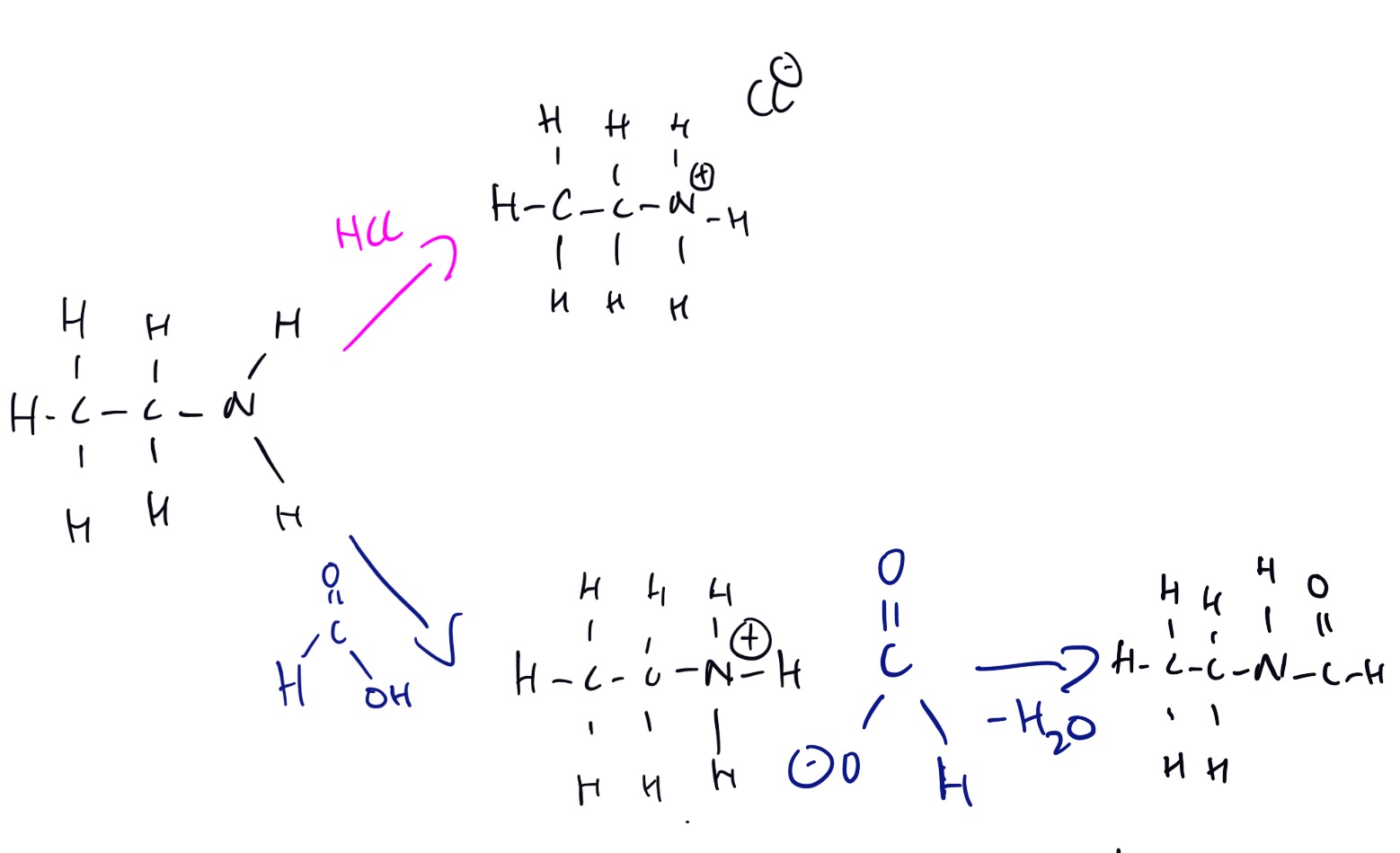
The video below explains how to identify, name and properties of amines.
Carboxylic acids can be produced by using the following reactions below:
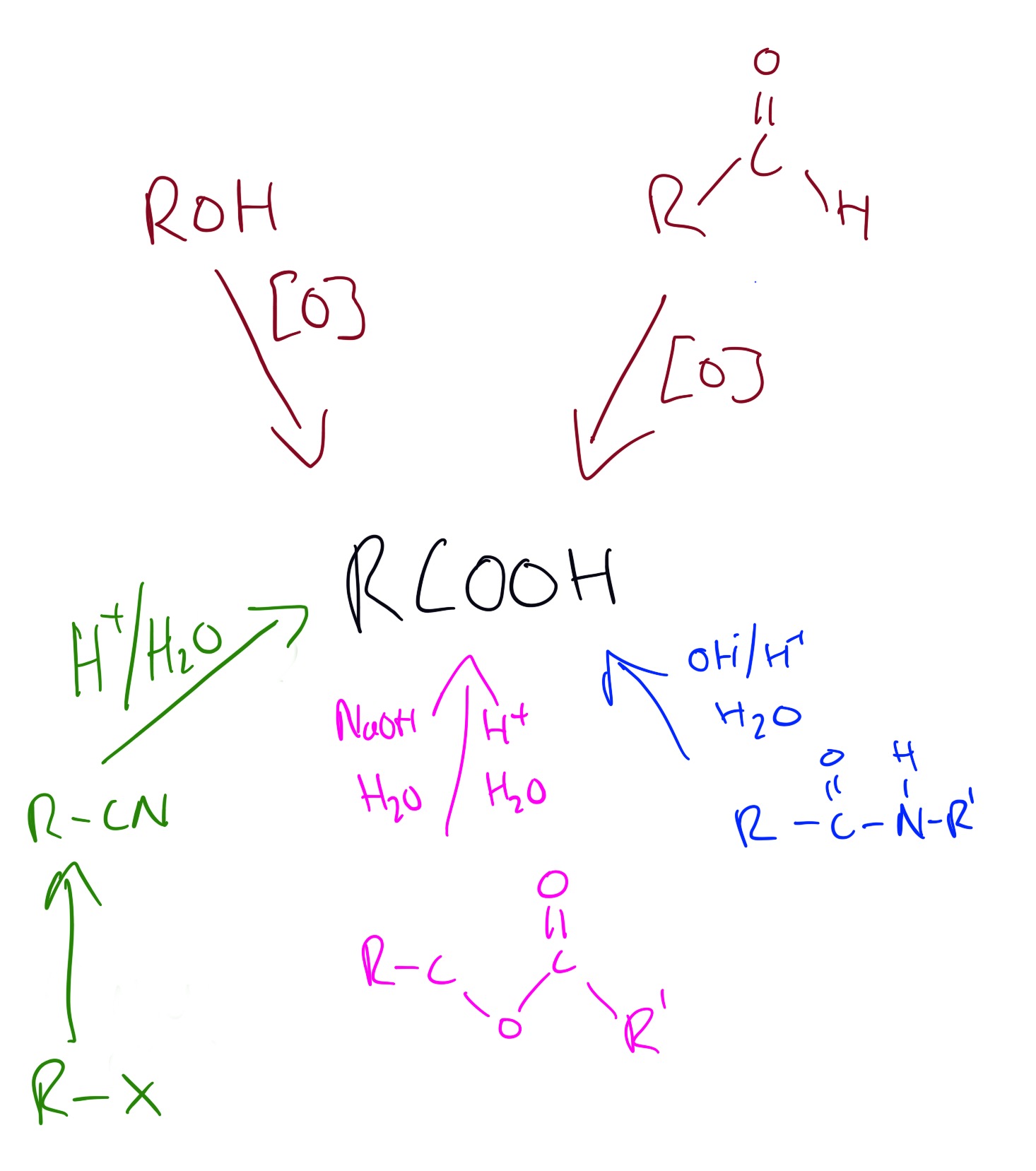
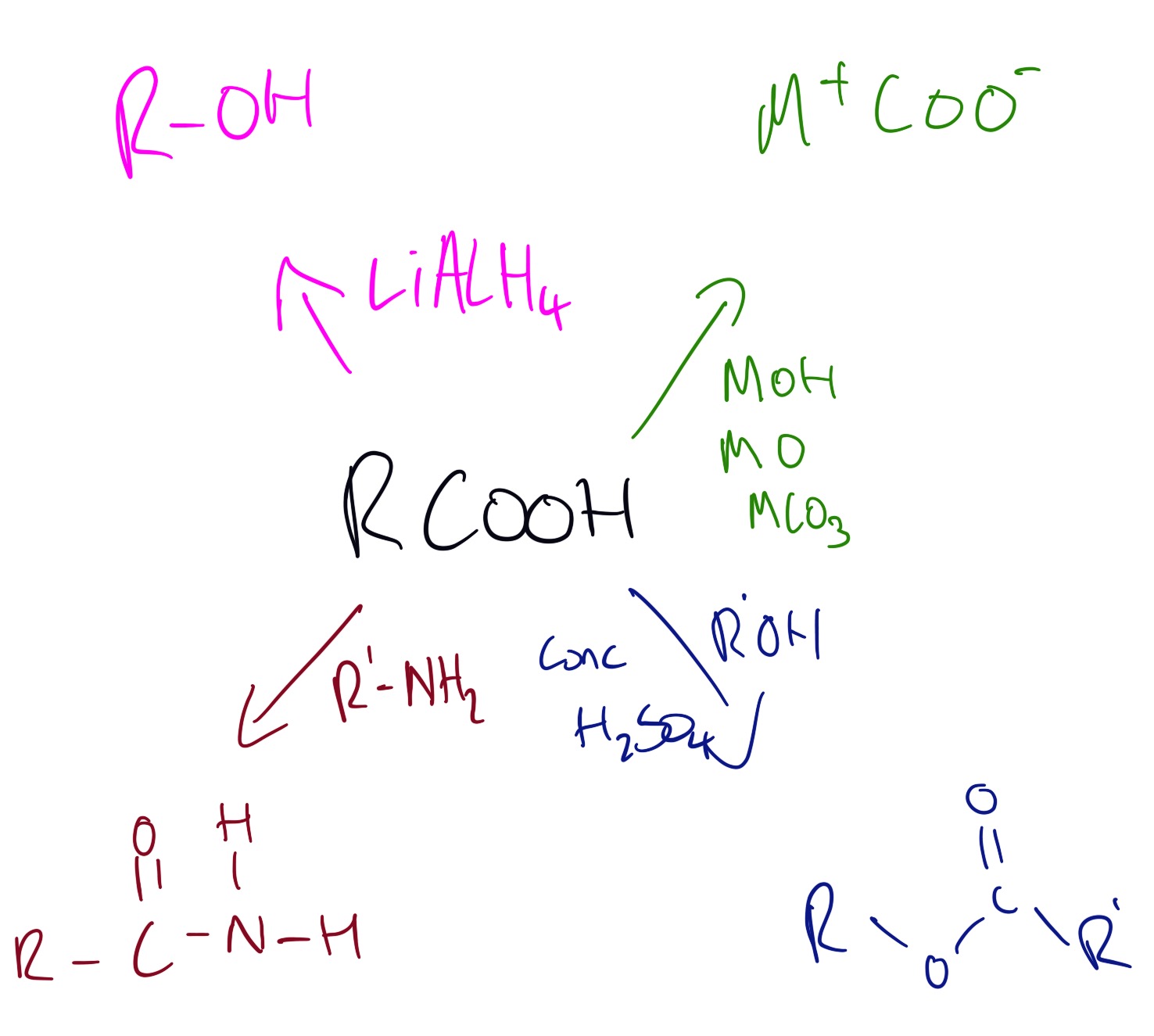
Explanations of the above reactions can be seen in the video below.
The electrophilic substitution reactions of benzene are shown below.
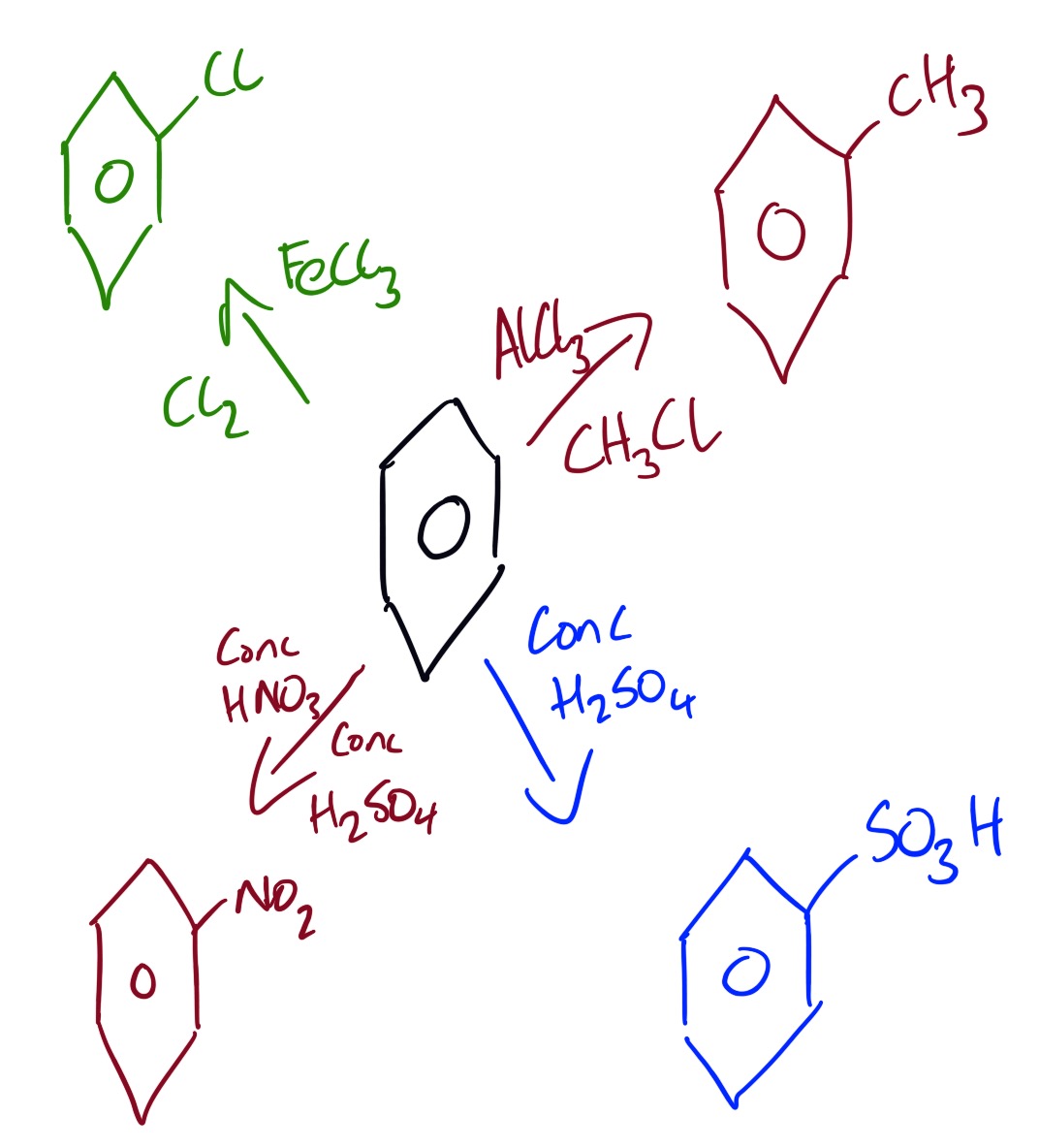
The video below explains the structure