Isomers are organic compounds which have the same molecular formula but different structural formulae.
Isomerism arises whenever there is more than 1 way to organise a given number of atoms. This is very common in organic chemistry. When talking about isomers we are always talking about 2 or more molecules. One molecule isn't an isomer by itself but can be an isomer of one or more other molecules.
What class of compounds do A and B belong to?
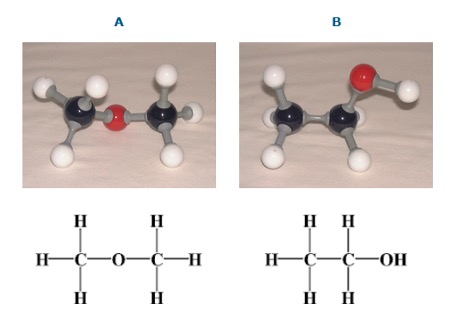
These are 2 isomers:
These 2 isomers belong to different homologous series but the difference isn't always this obvious.
Structural isomers have different physical and chemical properties and can even belong to different homologous series.
In stereoisomerism, the molecules have the same molecular formula and the same structural formula. However, in each molecule, the atoms have different 3D arrangements in space which makes them non-superimposable. No matter which way you twist and turn the molecules, one isomer can't fit exactly on top of the other.
There are 2 main types of stereoisomerism:
Geometric isomerism can arise when there is a lack of free rotation around a bond, often a C=C bond.
There are 2 geometric isomers of 1,2-dichloroethene:
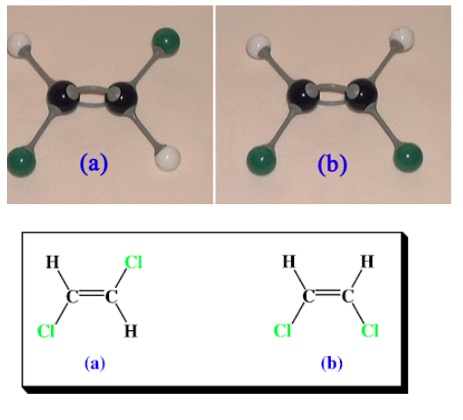
They exist because rotation around the C=C is very difficult.
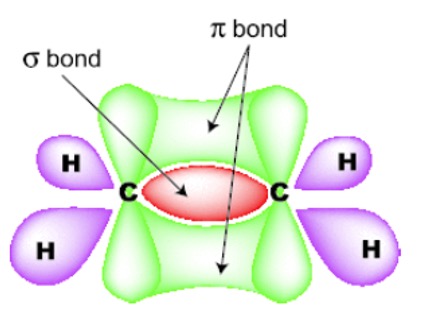
C=C consists of a sigma bond and a pi bond. Rotation is restricted because this would involve breaking the pi bond. The 2 geometric isomers are not superimposable.
Geometric isomers caused by the restricted rotation around a bond are distinguished by using the terms 'cis' and 'trans'.
Cis - both groups are on the same side of the double bond.
Trans - the groups are on opposite sides of the double bond.
In the diagram above, molecule (a) is known as 'trans-1-2-dichlormethane' and molecule (b) is known as 'cis-1-2-dichlormethane'.
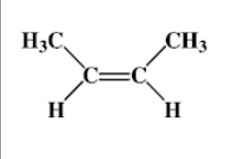
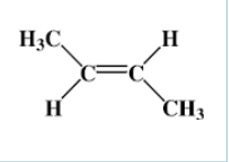
Similar situations can occur with alkenes containing 4 or more carbons.
What are the names of the molecules below?
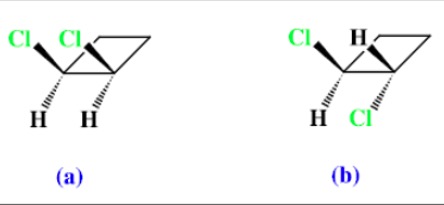
Geometric isomerism can also arise in disubstituted cycloalkanes.
Name the two molecules below:
The differences in melting point for each pair of isomers can be explained in terms of the shapes of molecules. This determines how closely the molecules pack together.
Sometimes geometric isomers can show differences in chemical properties although this is much less common than for structural isomers.
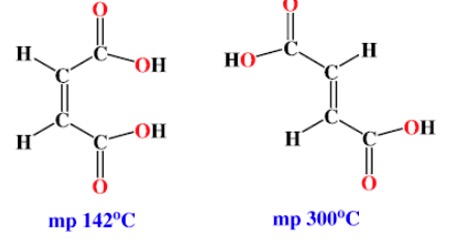
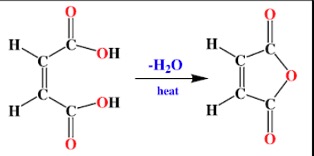
Cis-butenedioic acid readily eliminates water on heating. The product is known as a cyclic anhydride.
Optical isomerism can occur in compounds with 4 different groups arranged around a carbon atom. This leads to chiral molecules that are non-superimposable mirror images of each other.
Your hands are mirror images of each other.
Many chemical compounds can exist in 2 mirror image forms.
Right and left hands can't be superimposed on top of each other so that all the fingers coincide and are therefore not identical.
These are said to be chiral.
The hands and compounds have no centre of symmetry, plane of symmetry or axis of symmetry.
Chirality arises from a lack of symmetry, lack of symmetry is known as asymmetry.
Optical isomerism occurs in carbon compounds and other compounds e.g. transition metal compounds.
The 4 bonds around a carbon are arranged tetrahedrally.
If there are 4 different groups around the C it is asymmetric and can exist in 2 isomeric forms.
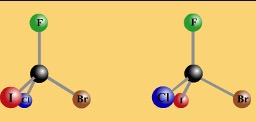
The tetrahedral C with 4 different groups is asymmetric.
Molecules containing one or more asymmetric carbon atoms are usually also asymmetric.
Asymmetric atoms or molecules are described as chiral with the carbon atom being called the chiral centre.
These molecules will differ from each other only in that they are mirror images of each other and exhibit optical activity.
These are called enantiomers or optical isomers.
A mixture containing equal amounts of each enantiomer is known as a racemic mixture.
Optical isomerism is hugely important in biological systems.
In most biological systems only 1 optical isomer of each organic compound is present. E.g. when amino acid alanine is synthesised in the lab, a mixture of 2 isomers is produced. When produced in nature only 1 of the forms is seen.
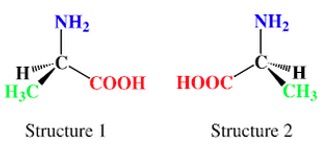
The proteins in our bodies are built using only 1 of the enantiomeric forms of amino acids.
Since amino acids are the monomers in proteins such as enzymes, enzymes will also be chiral.
The active site of an enzyme can only operate on one type of optical isomer. E.g. in the action of penicillin.
Penicillin works by preventing the formation of peptide links involving the enantiomer of alanine present in the cell walls of bacteria.
This enantiomer is not found in humans. Penicillin can therefore attack and kill the bacteria but not harm the human host.
Enantiomers behave in the same way in ordinary test tube reactions.
Physical properties, mp, bp, density, are also identical.
Enantiomers behave differently when in the presence of other chiral molecules.
E.g. enantiomers can taste different due to the chiral nature of taste buds.
One enantiomeric form of amino acids taste sweet, the other isomer can taste bitter.
Chemicals with enantiomeric forms can smell different.
e.g. Limonene - enantiomer one smells of lemons the other of oranges
Some substances will allow light vibrating in in only a single plane to pass through them producing plane polarised light. Polaroid sunglasses use this property to reduce glare.
Enantiomers have an opposite effect on plane polarised light. This is why they are said to be optically active. A polarimeter can be used to detect this effect.
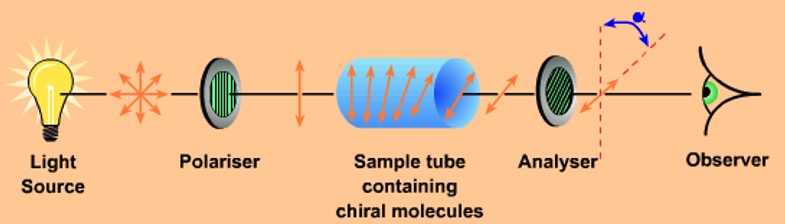
A compound is said to be optically active if, when inserted into a beam of plane polarised light, it rotates the light and causes it to vibrate in a different plane.
Enantiomers have this property, since solutions of one kind rotate plane polarised light in a clockwise direction and the other enantiomer rotates plane polarised light by the same amount in the opposite direction.
Many medicines are produced as a mixture of enantiomers, only 1 of which is pharmacologically active. It is often too expensive to separate the enantiomers.
One of the enantiomers of salbutamol used for asthma is 68 times more effective than the other.
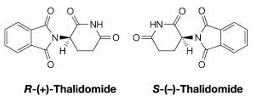
However, care must be taken when using drugs with enantiomeric forms as this has led to tragedy in the past e.g. thalidomide.
Thalidomide was first marketed to health professionals as a sedative. However, its use rapidly increased amongst pregnant women due to the drug's ability to alleviate morning sickness.
Soon after its rise in popularity, medical professionals began to note a series of congenital mutations in children born from mothers who used the drug during pregnancy, resulting in the thalidomide tragedy known today.