

Drugs - are substances that alter the biochemical processes in the body. Drugs that have beneficial effects are used in medicines.
Medicines - usually contains the drug plus other ingredients such as fillers to add bulk or sweeteners to improve the taste.
Drugs generally work by binding to specific protein molecules. These protein molecules can be found on the surface of a cell (receptor) or can be specific enzyme molecules within a cell.
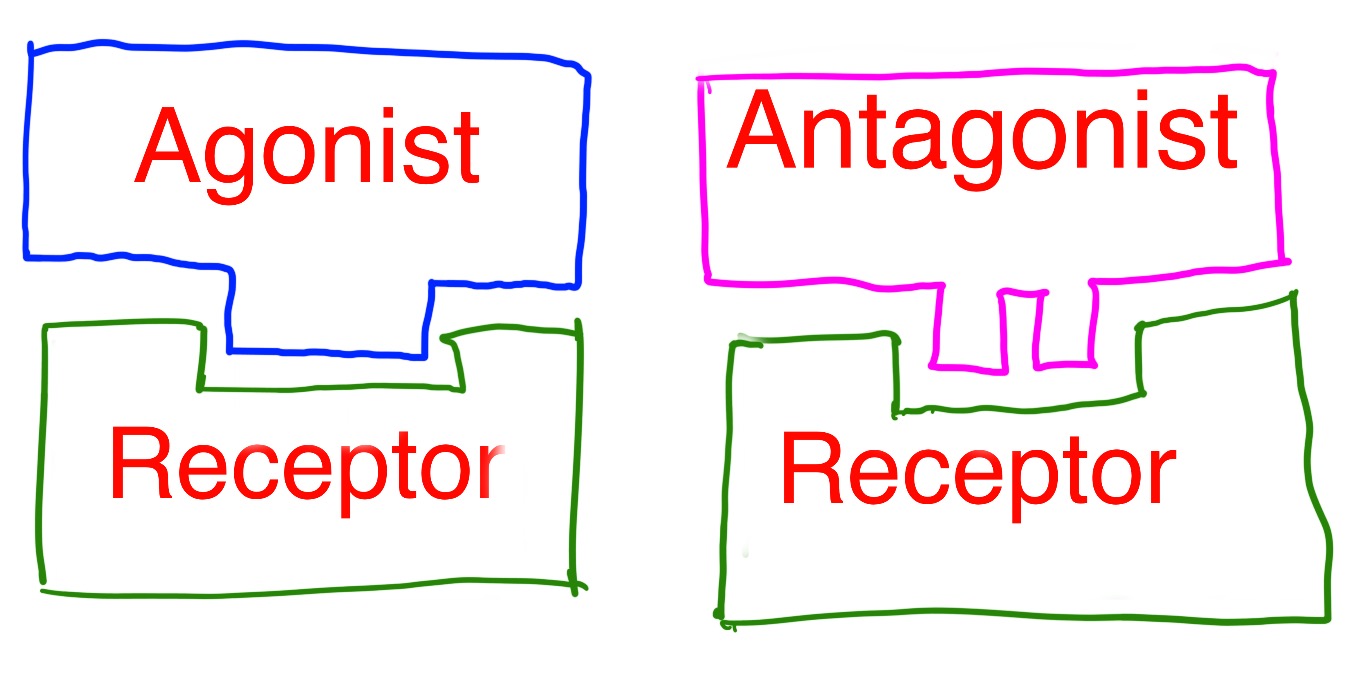
Drugs that act on receptors can be classified as agonists or antagonists.
Agonists mimic the natural compound and binds to the receptor molecules to produce a response similar to the natural active compound.
e.g. salbutamol in asthma inhalers.
Antagonists prevents the natural compound from binding to the receptor, and so blocks/inhibts the natural response from occurring.
e.g. propanolol used to treat high blood pressure.
Agonists and antagonists work by binding to receptors.
A receptor is a part of a very large protein molecule that has evolved naturally to interact with a specific small, biologically active molecule. The shape, size and structure of the receptor site are such that the biologically active molecule can bind reversibly with the receptor.
Agonists match the shape of the receptor and encourage a similar response.
Antagonists bind to the receptor but don't mimic the shape. This prevents a response being triggered and blocks the receptor.
Such binding, between functional groups in the active molecule and functional groups in the complex protein, involves weak forces such as hydrogen bonding and weak electrostatic interactions.
Many drugs that act on enzymes are classified as enzyme inhibitors and act by binding to the active site of the enzyme and blocking the reaction normally catalysed there.
The bonding at the receptor site is helpful for selecting the type of drug that will bind to the receptor.
Drugs can bind to receptors by ionic bonds on intermolecular forces of attraction.
The diagram below shows a drug with a negatively charged head group binding to a receptor with a positively charged head group.
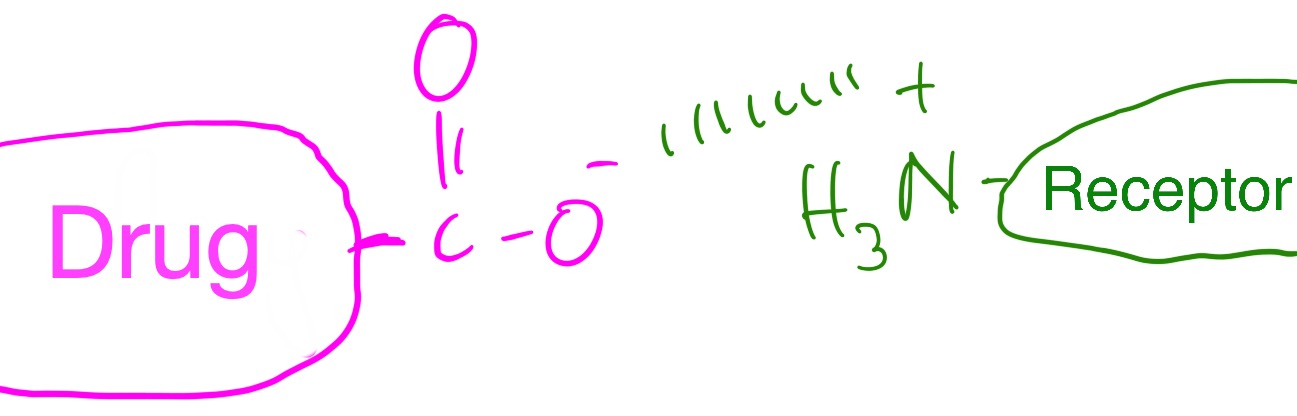
The diagram below shows a drug with a positively charged head group binding to a receptor with a negatively charged head group.
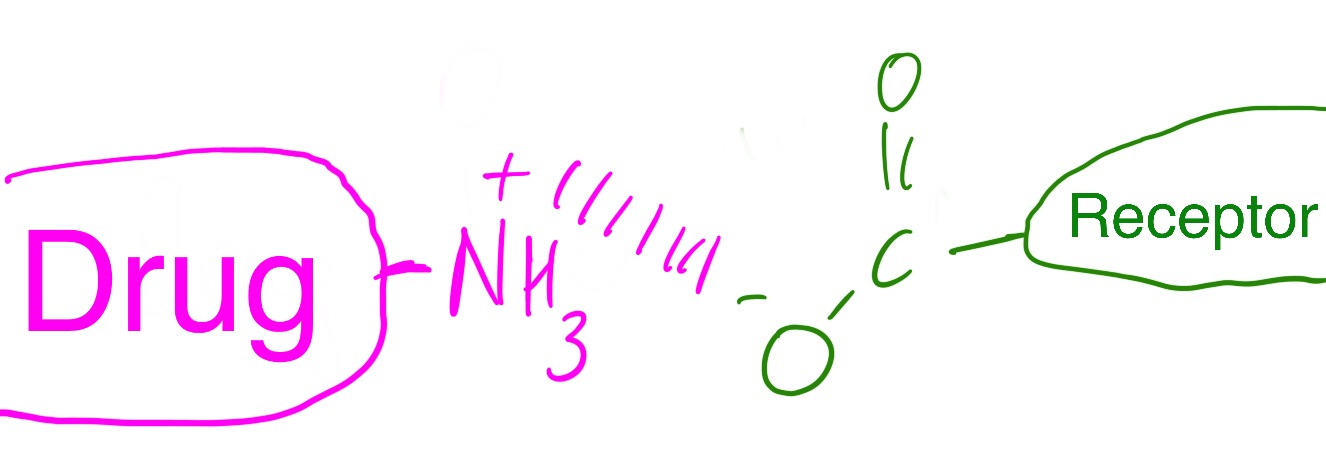
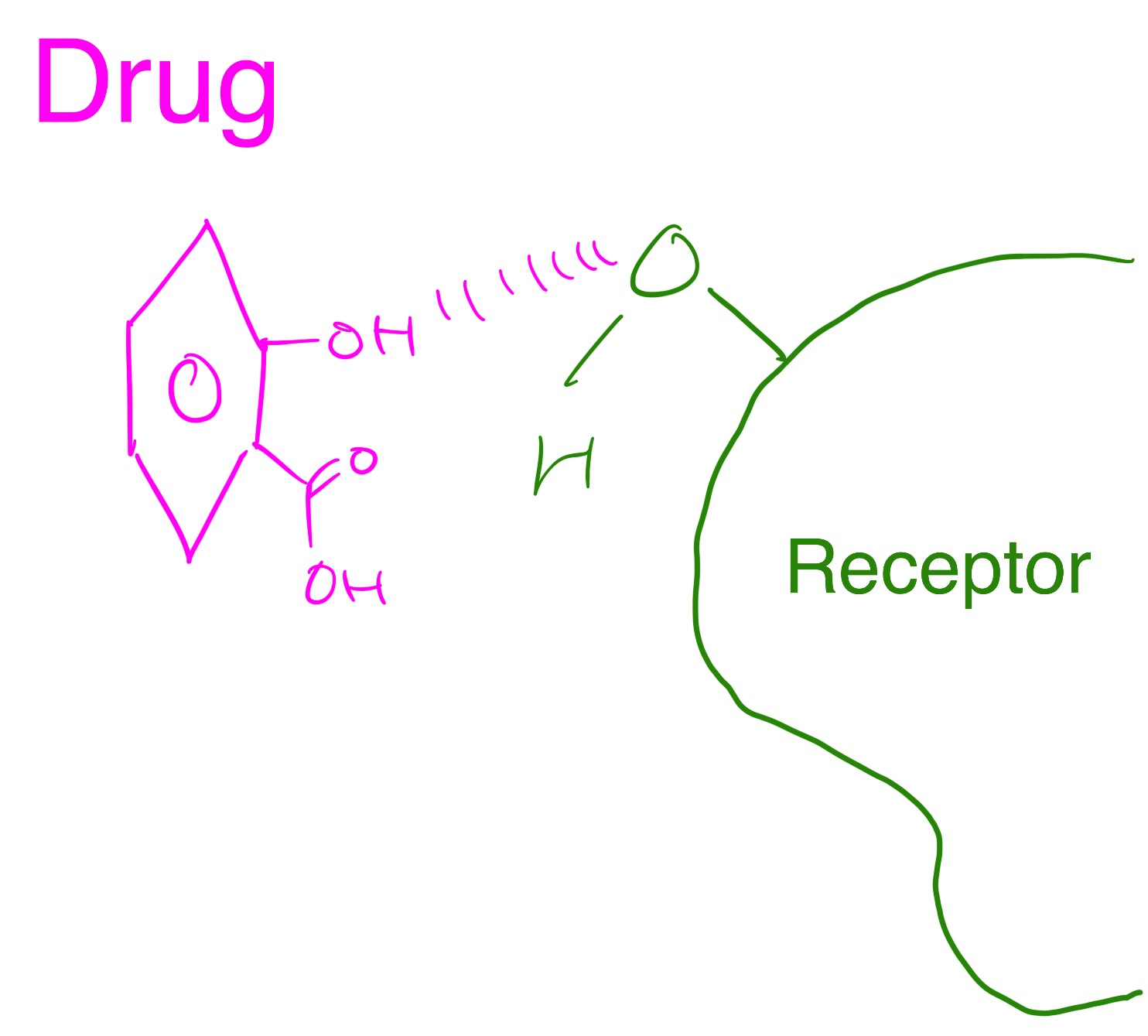
The diagram below shows a drug binding to a receptor through hydrogen bonding.
The structural fragment that is involved in the drug action is known as the pharmacophore. This can be identified by comparing structures different drugs that have similar effects on the body.
All of the above is summarised in the video below.
Percentage by mass is the mass of solute made up to 100 cm3 of solution.
% mass = (mass of solute ÷ mass of solution) x 100
A salt solution is made up of 40g of salt dissolved in 100g of water. Calculate the percentage mass of solute.
Percentage solution by volume is the number of cm3 of solute made up to 100 cm3 of solution.
% volume = (volume of solute ÷ volume of solution) x 100
A bottle of Vodka has 40 ml alcohol per 100 ml of solution. Calculate the % volume of ethanol in solution.
The unit ppm stands for parts per million and refers to 1 mg per kg or 1 mg per litre.
