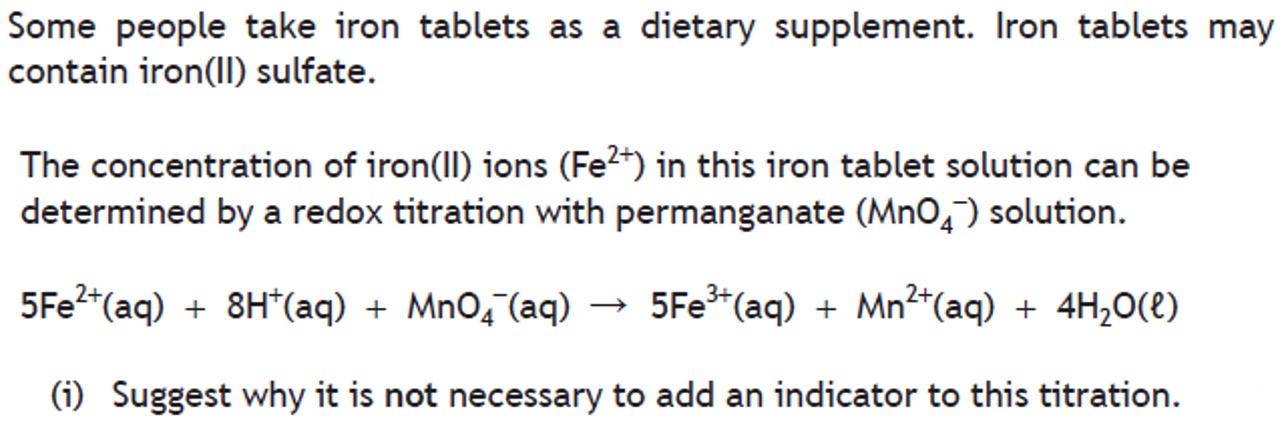 Chromatography is one technique widely used for analysis.
Chromatography derives from the Greek word 'Chroma' meaning colour and 'graphia' to mean writing.
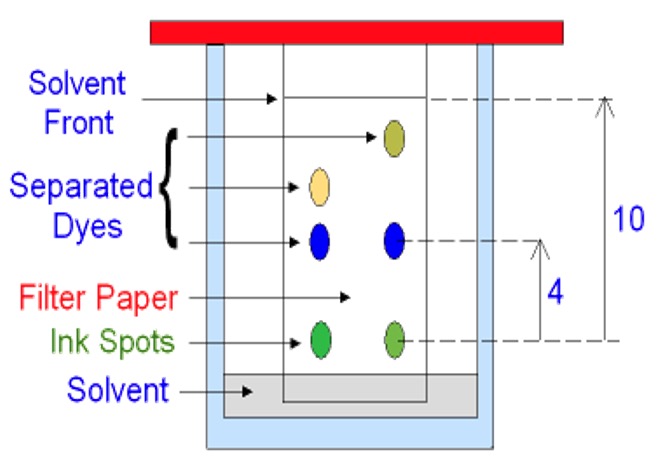
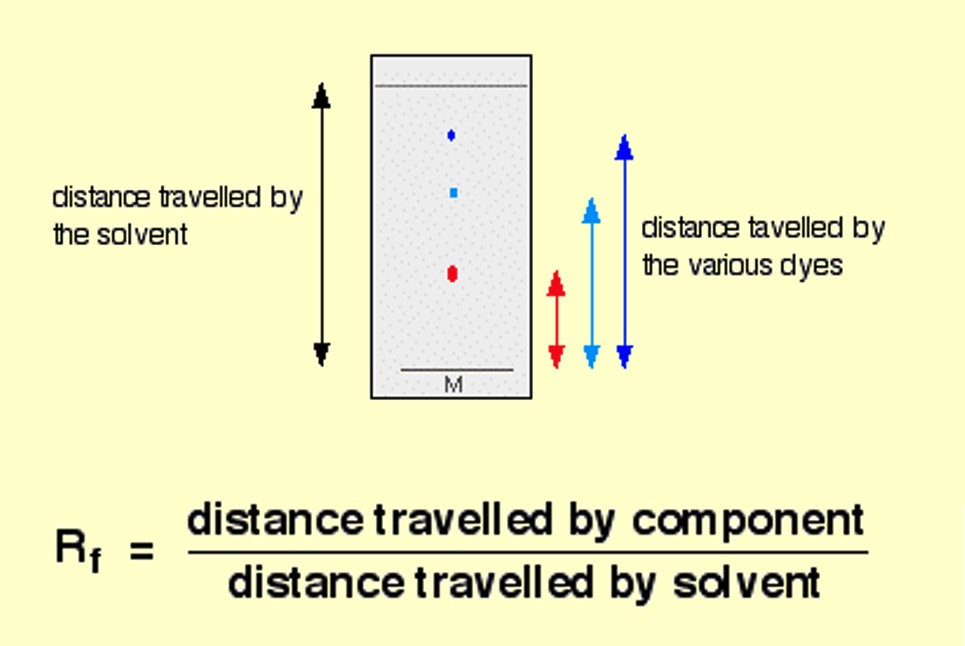
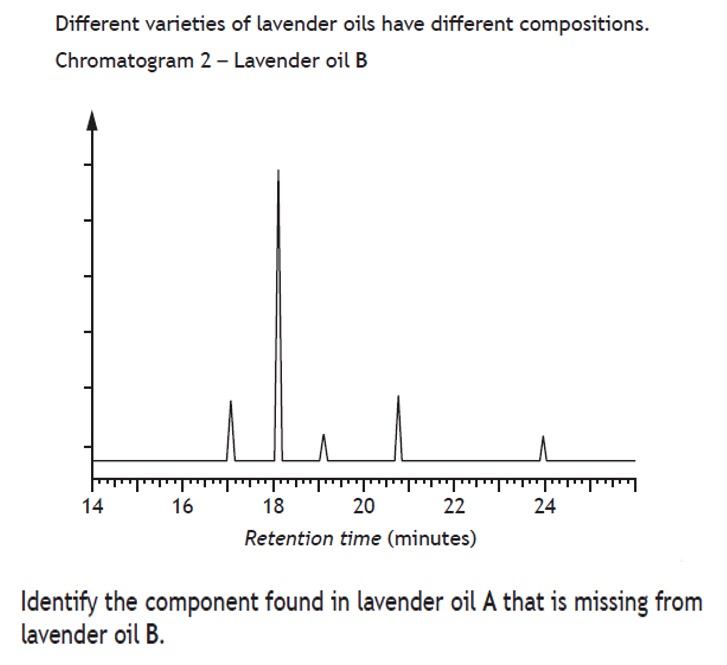
The results of a chromatography experiment can sometimes be presented graphically, showing an indication of the quantity of substance present on the y-axis and retention time of the x-axis.
Chromatography is one technique widely used for analysis.
Chromatography derives from the Greek word 'Chroma' meaning colour and 'graphia' to mean writing.
The results of a chromatography experiment can sometimes be presented graphically, showing an indication of the quantity of substance present on the y-axis and retention time of the x-axis.
Chromatography is a technique used to separate the components present within a mixture. Chromatography separates substances by making use of differences in their polarity or molecular size.
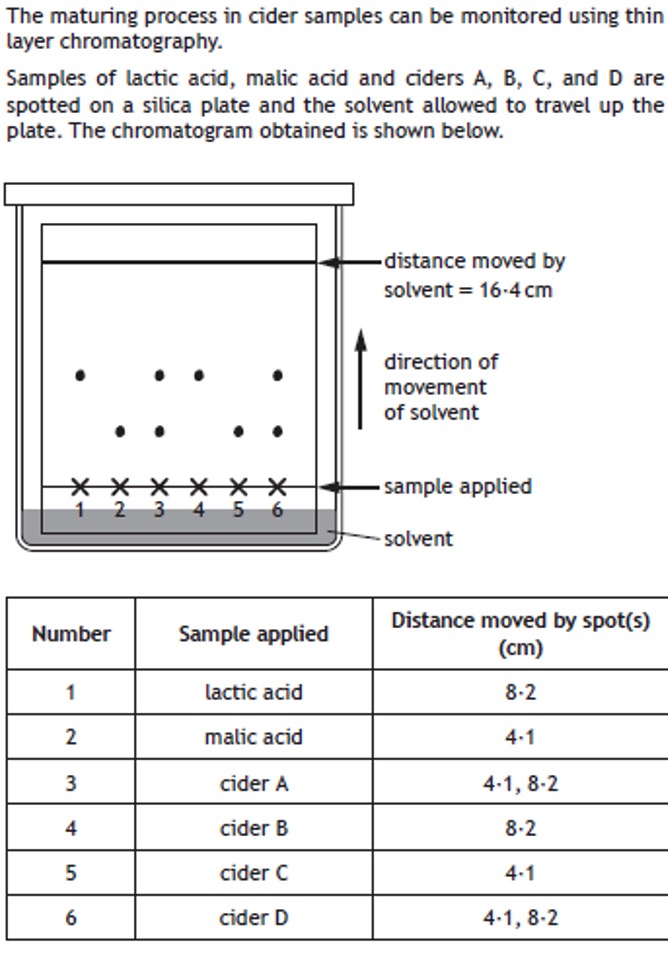


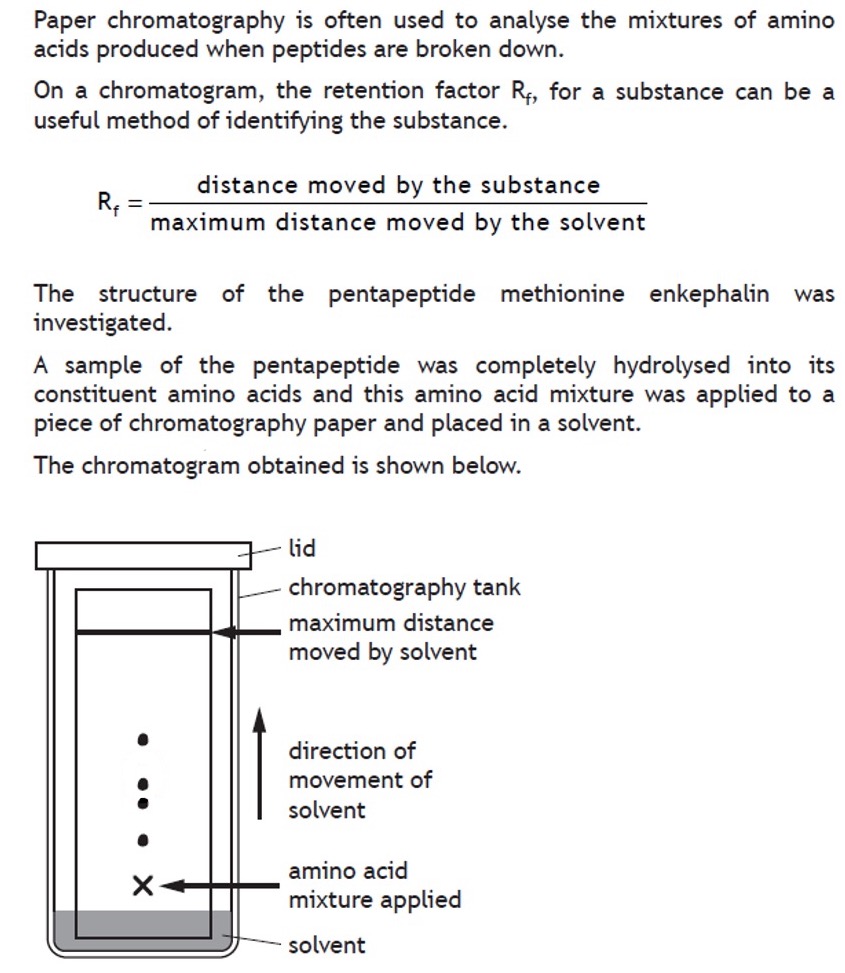


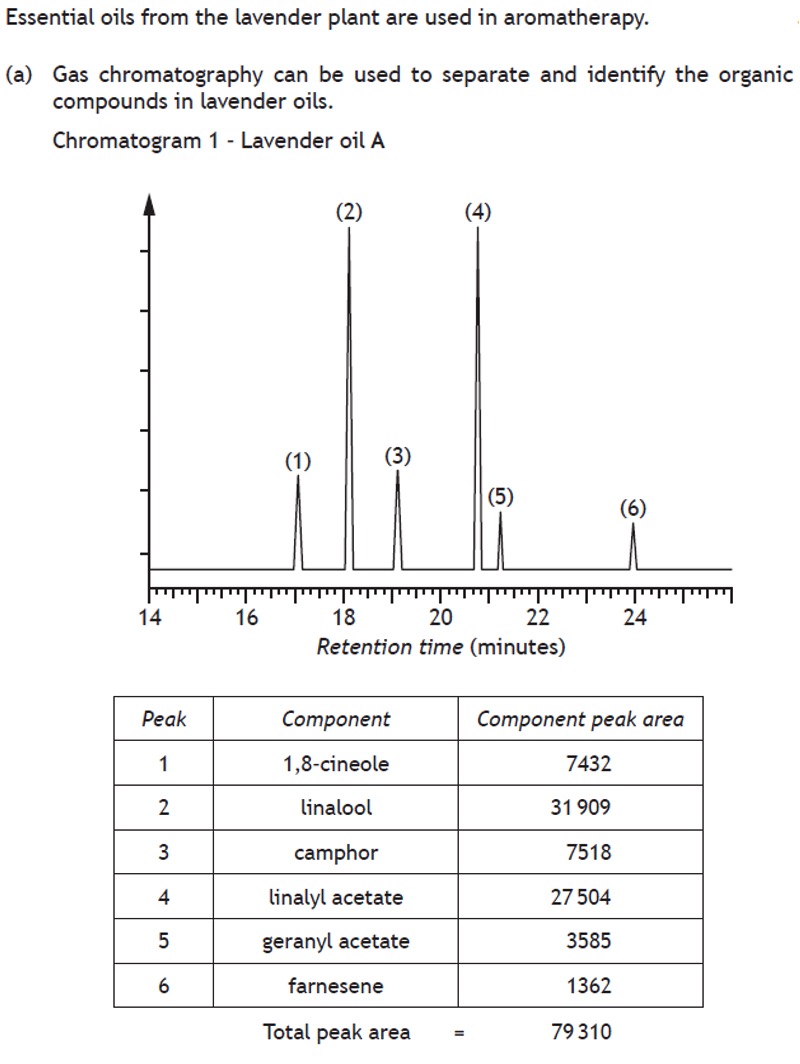
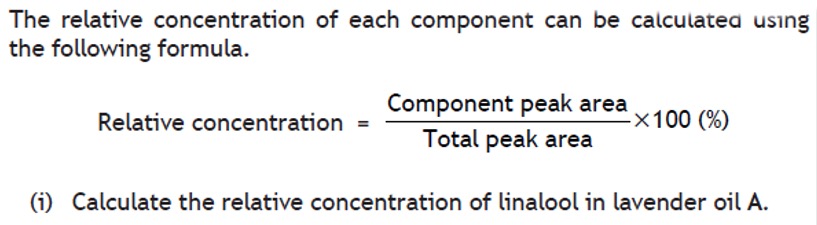
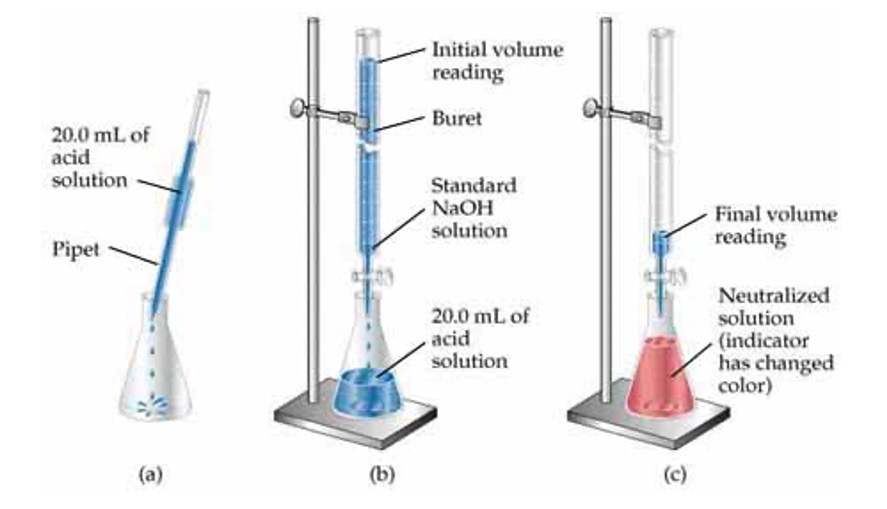
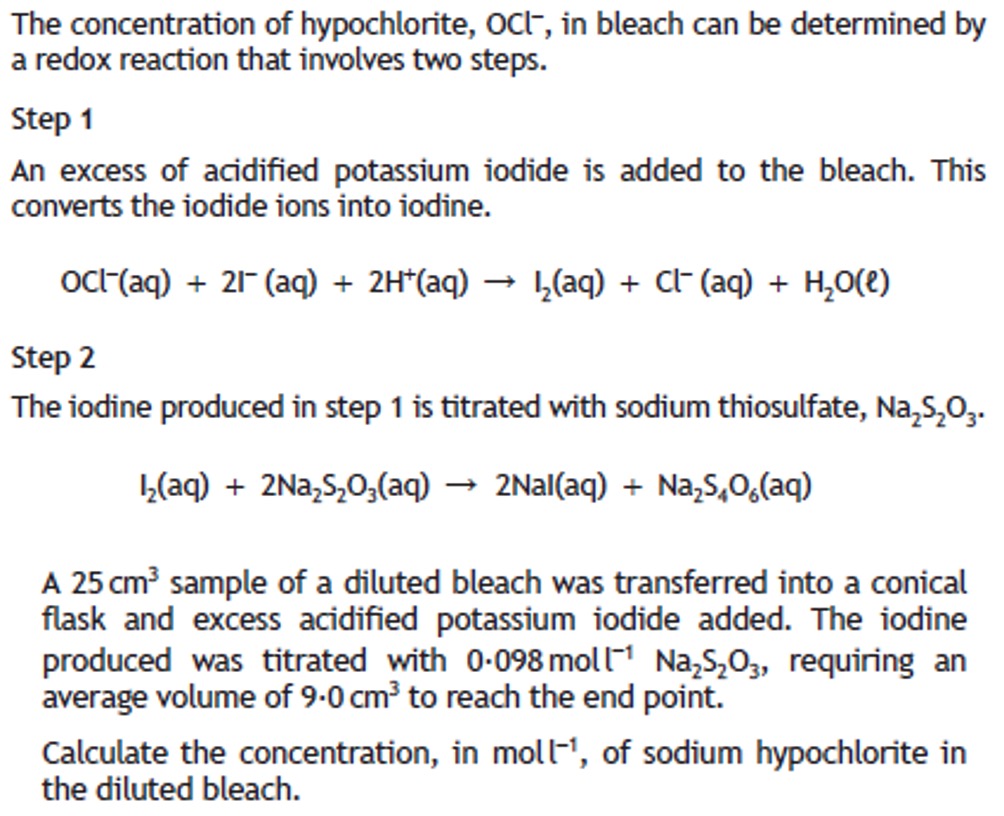
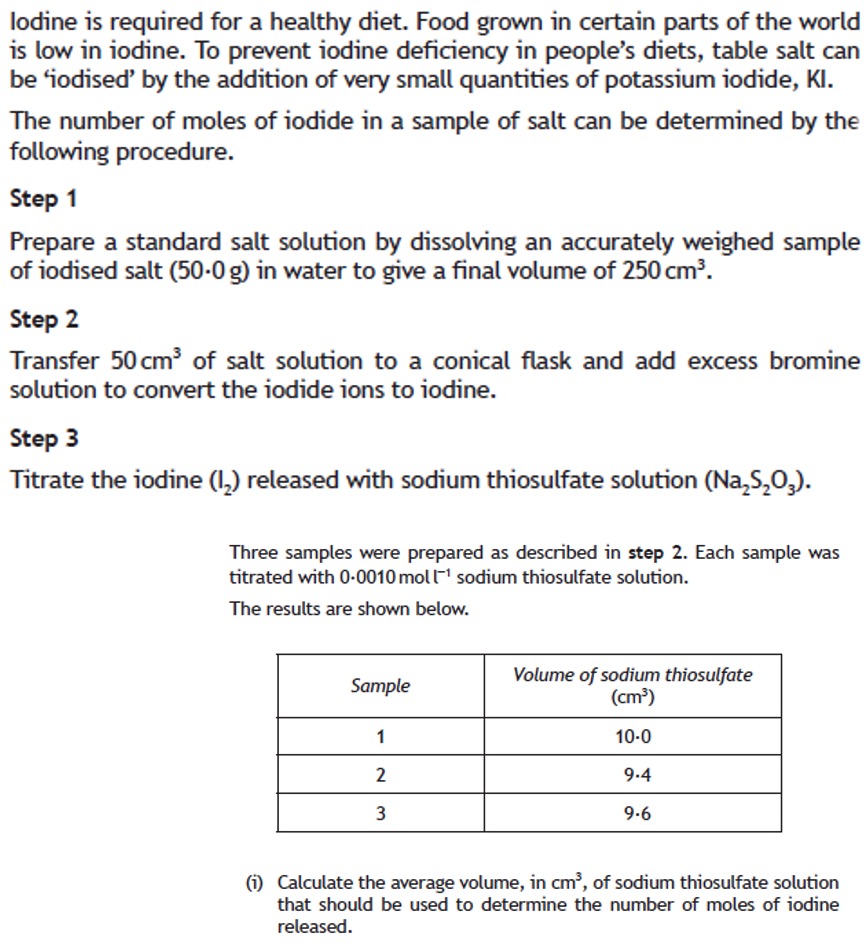
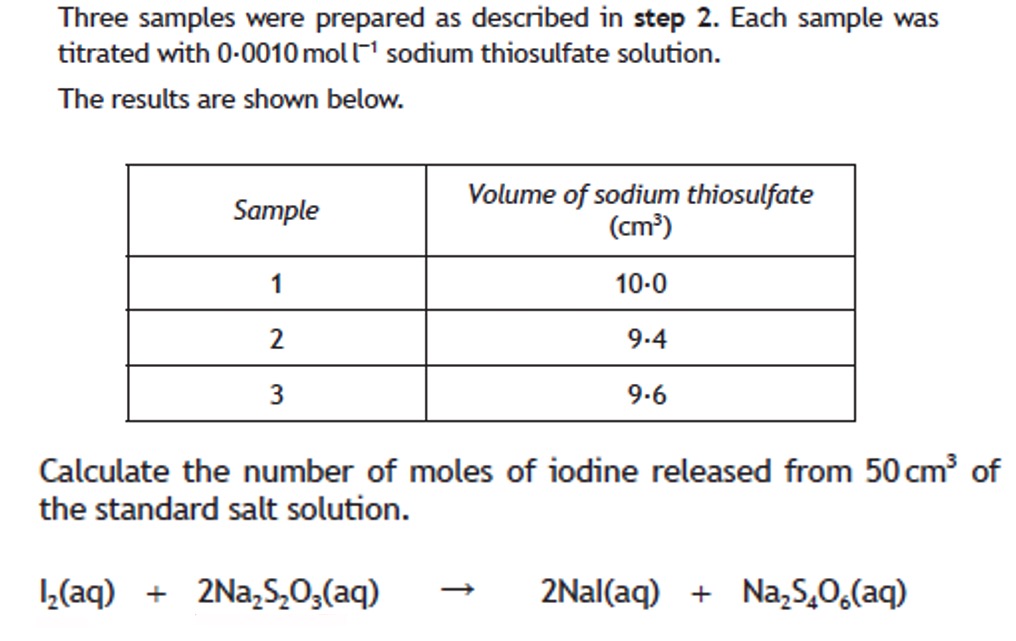
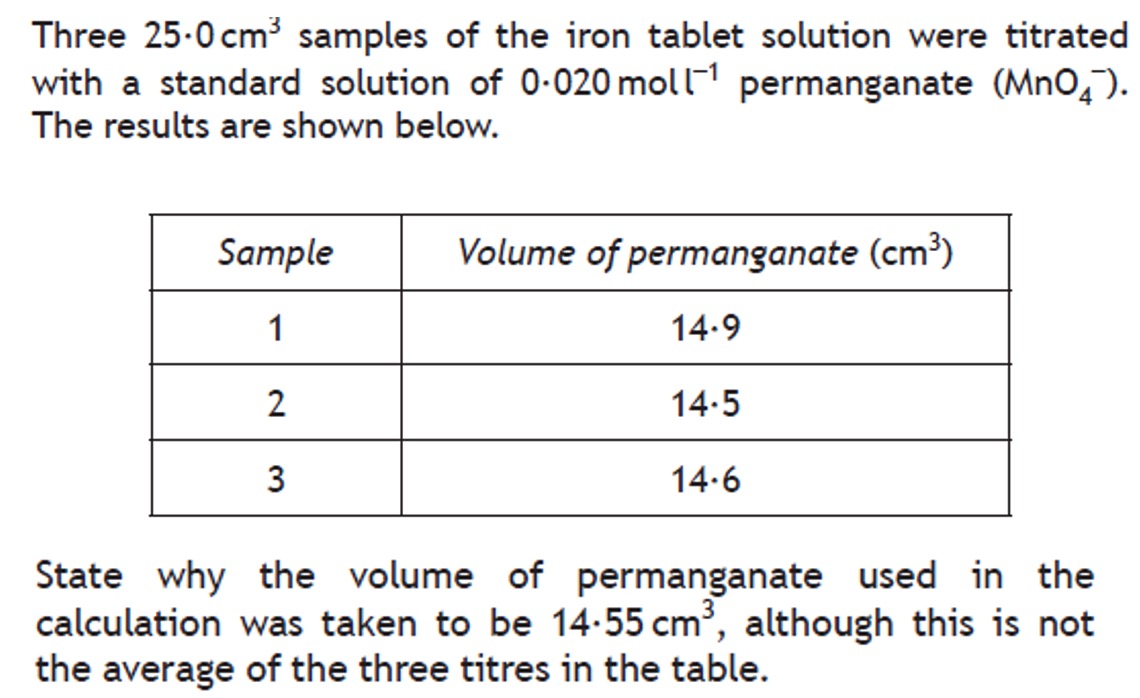
Volumetric analysis involves using a solution of accurately known concentration in a quantitative reaction to determine the concentration of another substance.