

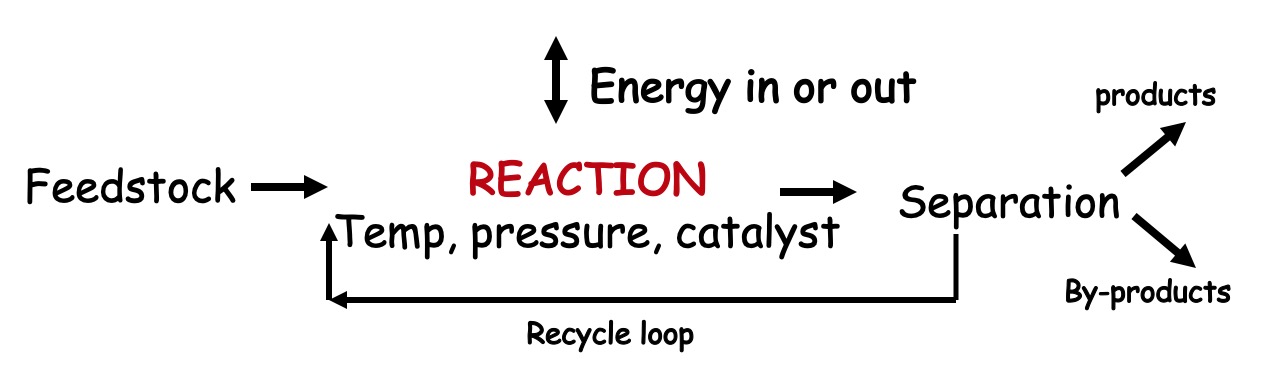
The key factors to take into consideration when manufacturing a new chemical are as follows:
Environmental considerations include:
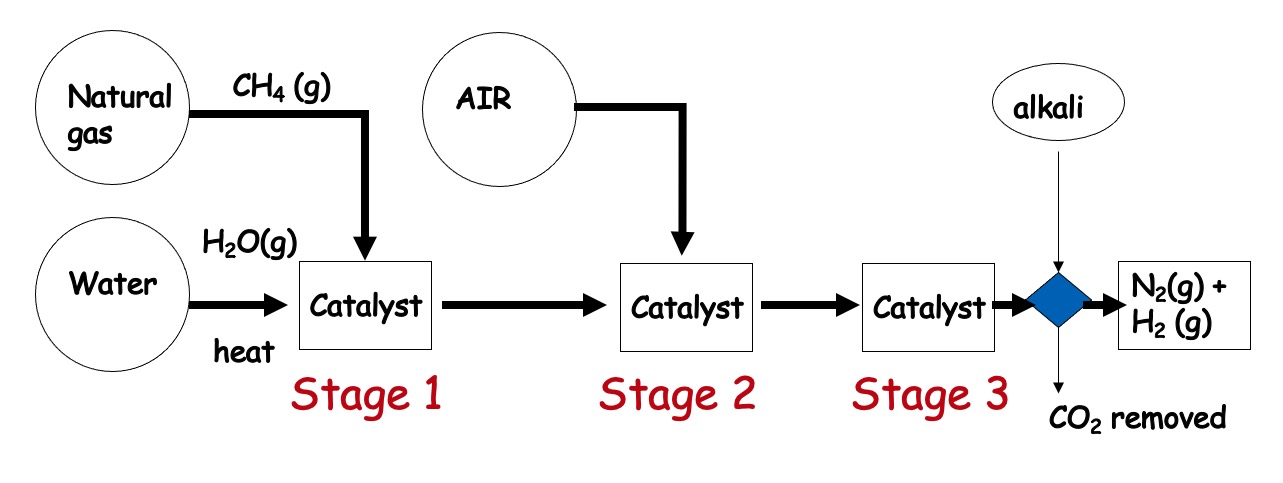
Ammonia is manufactured from N2 and H2. The nitrogen is available from the raw material, air. (something which is available naturally). The other feedstock for the manufacture of NH3 is hydrogen which is usually produced from methane.
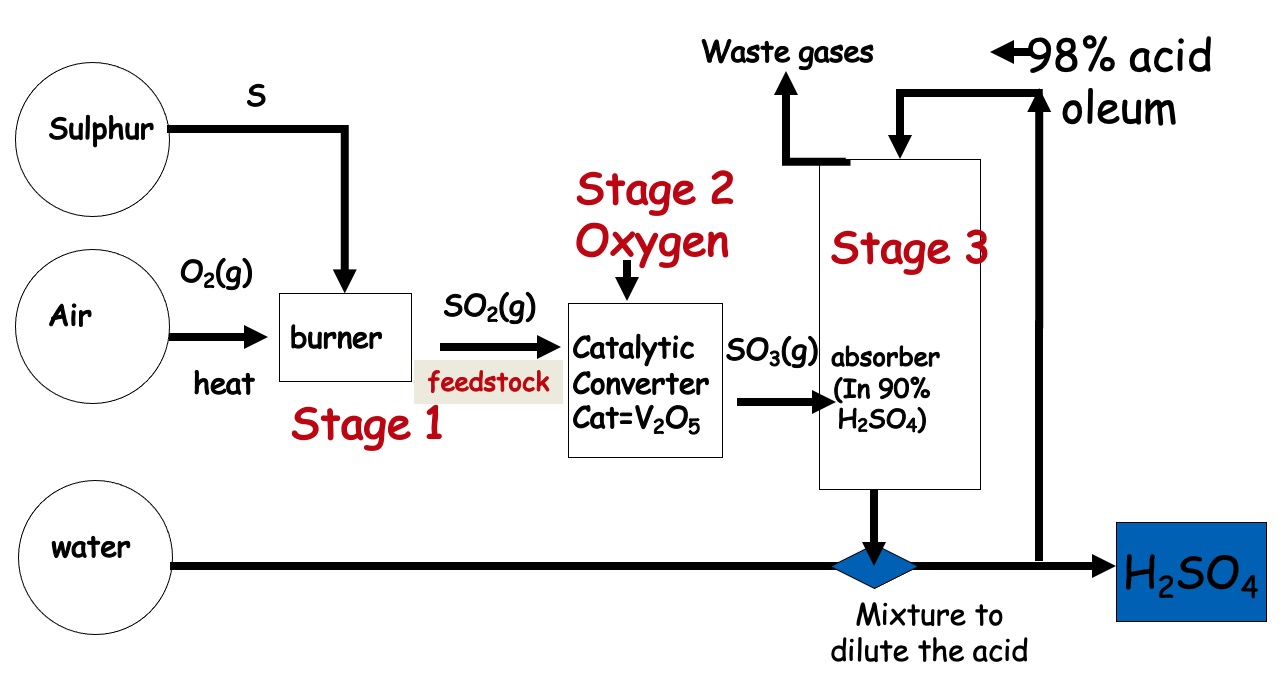
Sulfuric acid is manufactured by the Contact Process.
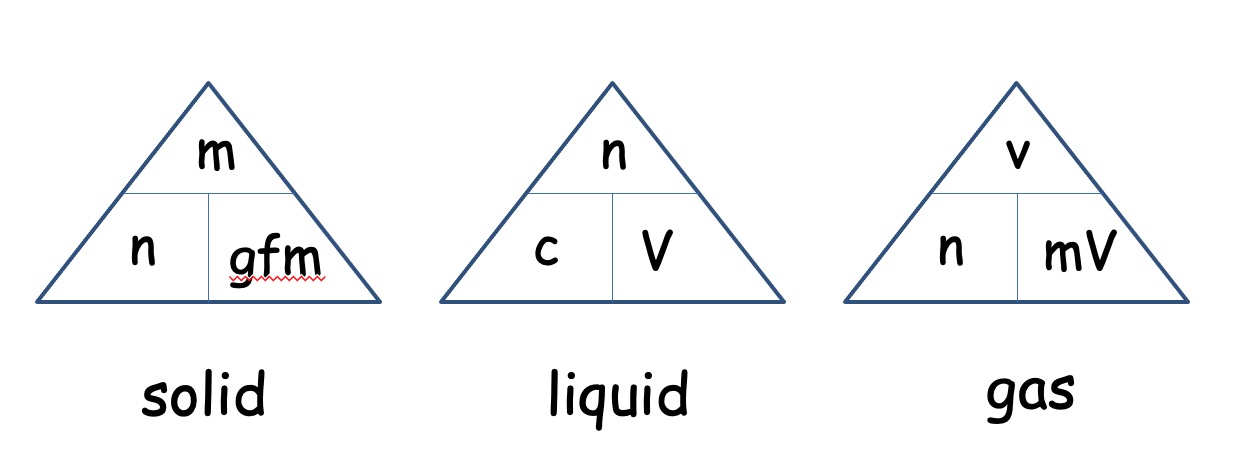
Using your knowledge from National 5 Chemistry you should be able to perform calculations from balanced equations.
The molar volume of a gas is its volume per mole, litre mol-1. It is the same for all gases at the same temperature and pressure. The value, though, is temperature and pressure dependent.
The molar volume of all gases is approximately 24 litre mol-1 at 20oC.
Calculate the volume of 0.025 moles of oxygen.
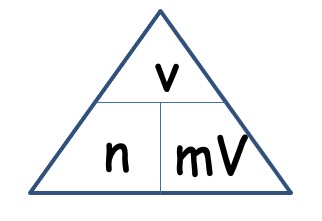
V = n x mV
V =
Calculate the number of moles in 72 litres of hydrogen.

n = V / mV
n =
As all gases have the same molar volume, volume can be taken as moles.
What volume of C02, is produced if 100 cm3 of O2 is used to completely to burn some CH4 gas?
1. What is the mass of steam in 180 cm3 of the gas, when the molar volume is 24 litres mol-1?
2. The equation below shows the reaction between calcium carbonate and hydrochloric acid.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
20g of calcium carbonate reacts with excess hydrochloric acid.
Calculate the volume of carbon dioxide gas formed. (Take the molar volume to be 24.0 litre mol-1)
3. Calculate the number of moles in 0.36 litres of argon (molar gas volume = 24 litres mol-1 ).
4. Calculate the volume of 0.04 moles of CO2. (molar gas volume = 24 litres mol-1 ).
You can use the relative numbers of moles of substances, as shown in balanced equations, to calculate the amounts of reactants needed or the amounts of products produced.
A limiting reactant is the substance that is fully used up and thereby limits the possible extent of the reaction. Other reactants are said to be in excess.
In order to ensure that a costly reactant and the reactant is converted into product, an excess of the less expensive reactant can be used.
Which reactant is in excess when 10g of calcium carbonate reacts with 100cm3 of 1 mol l-1 hydrochloric acid?
1.2g of magnesium was added to 100cm3 2 mol l-1 hydrochloric acid. Calculate the reagent in excess.
For each of the following reactions calculate which reagent is in excess and make a statement as to why.
1. 4.86g magnesium added to 250cm3 2 mol l-1 hydrochloric acid.
2. 2.7g aluminium added to 200cm3 1 mol l-1 hydrochloric acid.
3. 2.43g magnesium added to 200cm3 1 mol l-1 sulphuric acid.
4. 3.27g zinc added to 100cm3 0.2 mol l-1 hydrochloric acid.
In the following calculations you have to find which reactant is the limiting reagent to answer the question.
5. What mass of calcium oxide is formed when 0.4 g of calcium reacts with 0.05 mole of oxygen?
2Ca + O2 → 2CaO
What mass of hydrogen is formed when 3.27g of zinc is reacted with 25cm3 of 2 mol l-1 hydrochloric acid?
Zn + 2HCl → ZnCl2 + H2
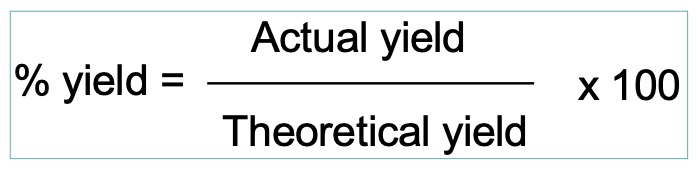
Percentage yield can be used to measure the efficiency with which reactants are converted into the desired products.
For a particular set of reaction conditions, the percentage yield provides a measure of the degree to which the limiting reagent is converted into the desired product.
The 'theoretical yield' is the quantity of desired product obtained, assuming full conversion of the limiting reagent, as calculated from the balanced equation.
The 'actual yield' is the quantity of the desired product formed under the prevailing reaction conditions.

Very few chemical reactions have a yield of 100% because:
Careful planning and design of the equipment and reaction conditions can help keep % yield high.
Calcium carbonate is used to make calcium oxide for cement making.
CaCO3 → CaO + CO2
1 mole of CaCO3 should produce 1 mole of CaO.
Theoretical yield: 1 mole CaO = (1x 40.1 + 1x 16) = 56g
Actual yield: 48g

Percentage yield = 48/56 x 100 = 85.7%
1. In the reaction below, the theoretical yield was 10.7g but the actual yield was 4.5g. Calculate the percentage yield.
Be + 2HCl → BeCl2 + H2
2.In the reaction below:
TiS + H2O → H2S + TiO
What is the percentage yield of titanium (II) oxide if you start with 20g of titanium (II) sulphide and the actual yield of titanium (II) oxide is 12g?
3. In the reaction below:
U + 3Br2 → UBr6
What is the actual yield of uranium hexabromide if you start with 100g of uranium and get a percentage yield of 83%?

The atom economy measures the proportion of the total mass of all starting materials converted into the desired product in the balanced equation.
Reactions which have a high percentage yield may have a low atom economy value if large quantities of by-products are formed.
Compare these two industrial reactions. What do you notice about each one?
2Mg + O2 → 2MgO
CaCO3 → CaO + CO2
In the reaction above CO2 is a waste product.
Often, chemical reactions produce unwanted products along with the product you want.
Calculate the atom economy for the reaction below.
CaCO3 → CaO + CO2

mass of desired product = 56g
mass of reactant = 100g
% atom economy = (56 / 100) x 100 = 56%
1. What is the percentage atom economy for the following reaction for making hydrogen by reacting coal with steam?
C(s) + 2H2O(g) → CO2(g) + 2H2(g)
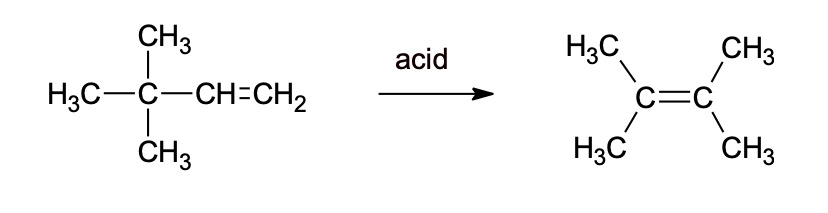
2. Calculate the percentage atom economy for the reaction below.
3. Hydrazine (N2H4) is used for rocket fuel. Calculate the atom economy for hydrazine production.
2NH3 + NaOCl → N2H4 + NaCl +H2O
Example 2: Molar Volume and Excess textbook Q16
Molar Volume and Excess - textbook Q20
Molar Volume and Excess - textbook Q21
Molar Volume and Excess - textbook Q22