Chemical reactions which take place in both directions are called reversible reactions.
In a closed system, reversible reactions attain a state of dynamic equilibrium when the rates of forward and reverse reactions are equal.
At equilibrium, the concentrations of reactants and products remain constant, but are rarely equal.
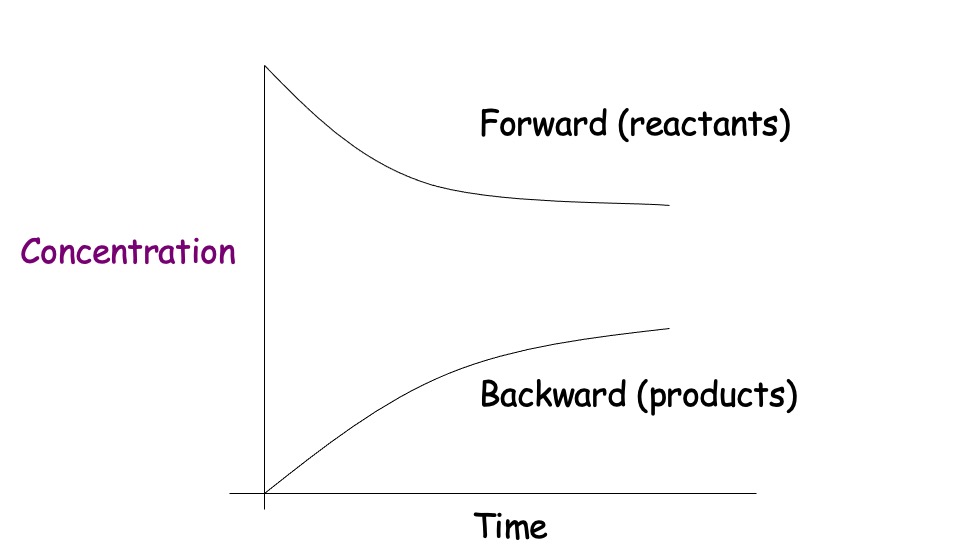
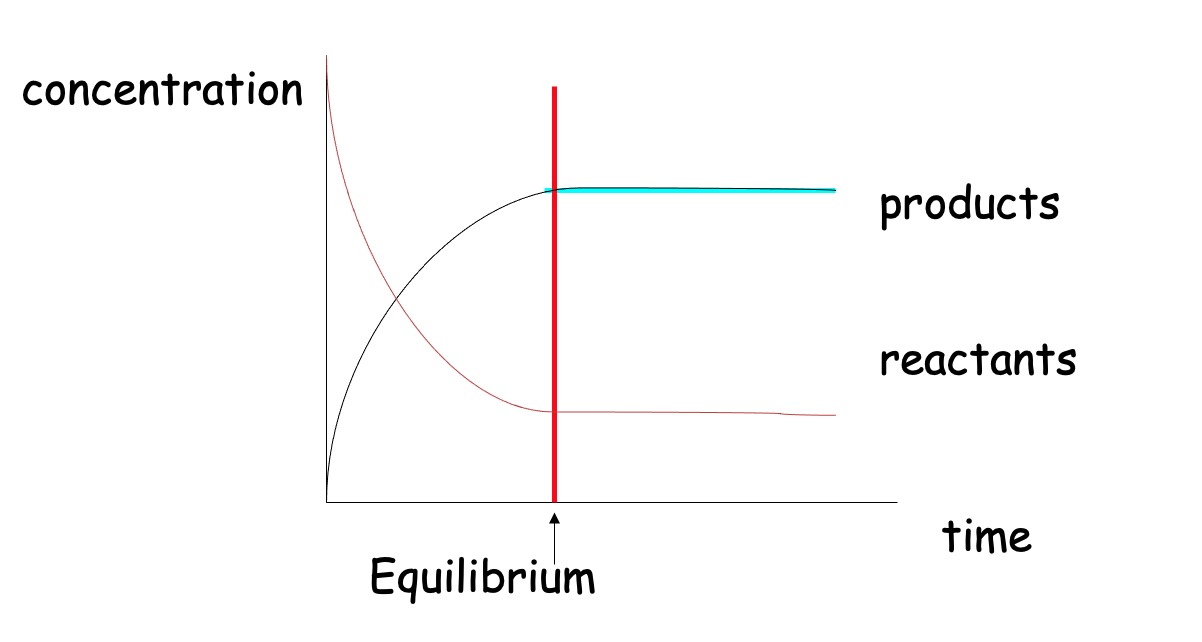
At equilibrium the concentration of products and reactants remains the same.
Iodine is soluble in both cyclohexane and KI solution. In cyclohexane it is purple. In KI solution, yellow-brown.
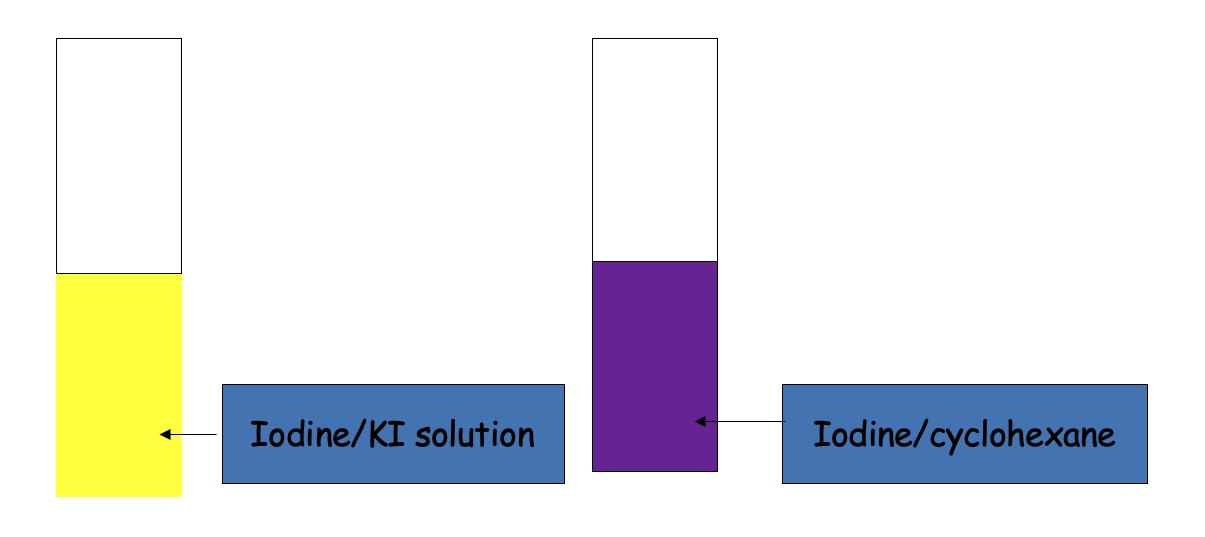
To each test tube, the other liquid/solution is added.
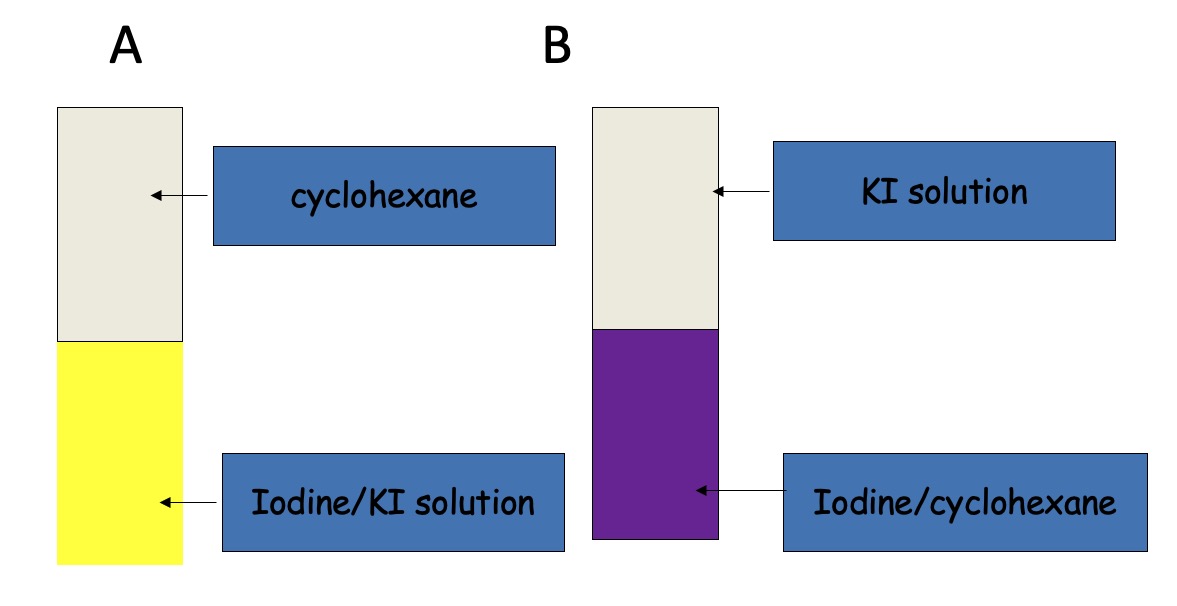
Final equilibrium is the same



An equilibrium will move to undo any change imposed upon it.
If the forward reaction is favoured we say the equilibrium has moved to the right.
If the reverse reaction is favoured we say the equilibrium has moved to the left.
To maximise profits, chemists employ strategies to move the position of equilibrium in favour of the products.
Heating a reversible reaction at equilibrium shifts the reaction in the direction of the ENDOTHERMIC REACTION
Cooling a reversible reaction at equilibrium shifts the reaction in the direction of the EXOTHERMIC REACTION
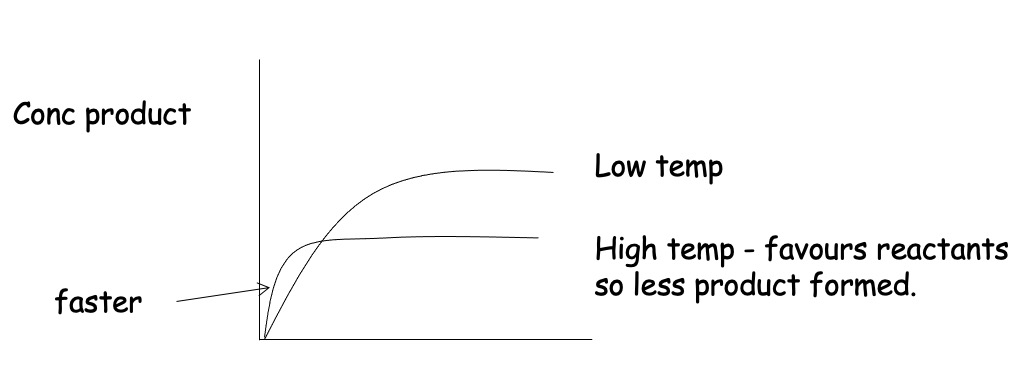
Consider the follwing Equilibrium:
Forward reaction is exothermic
Increasing temperature shifts equilibrium to the left.
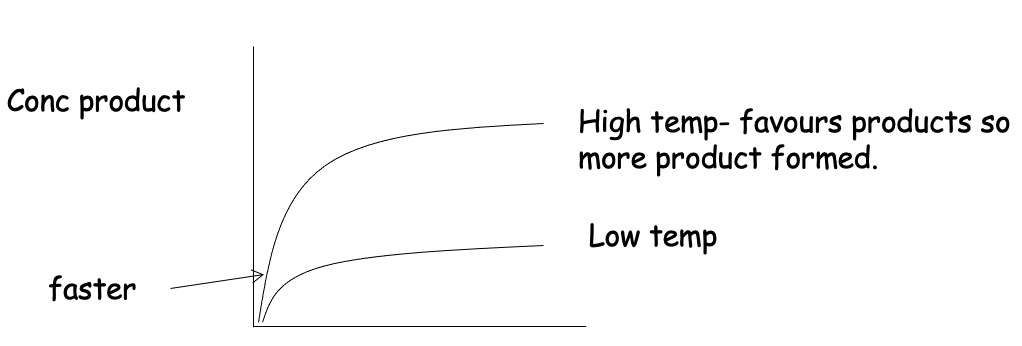
Consider the follwing Equilibrium:
Forward reaction is endothermic.
Increasing temperature shifts equilibrium to the right.
Consider the follwing Equilibrium:
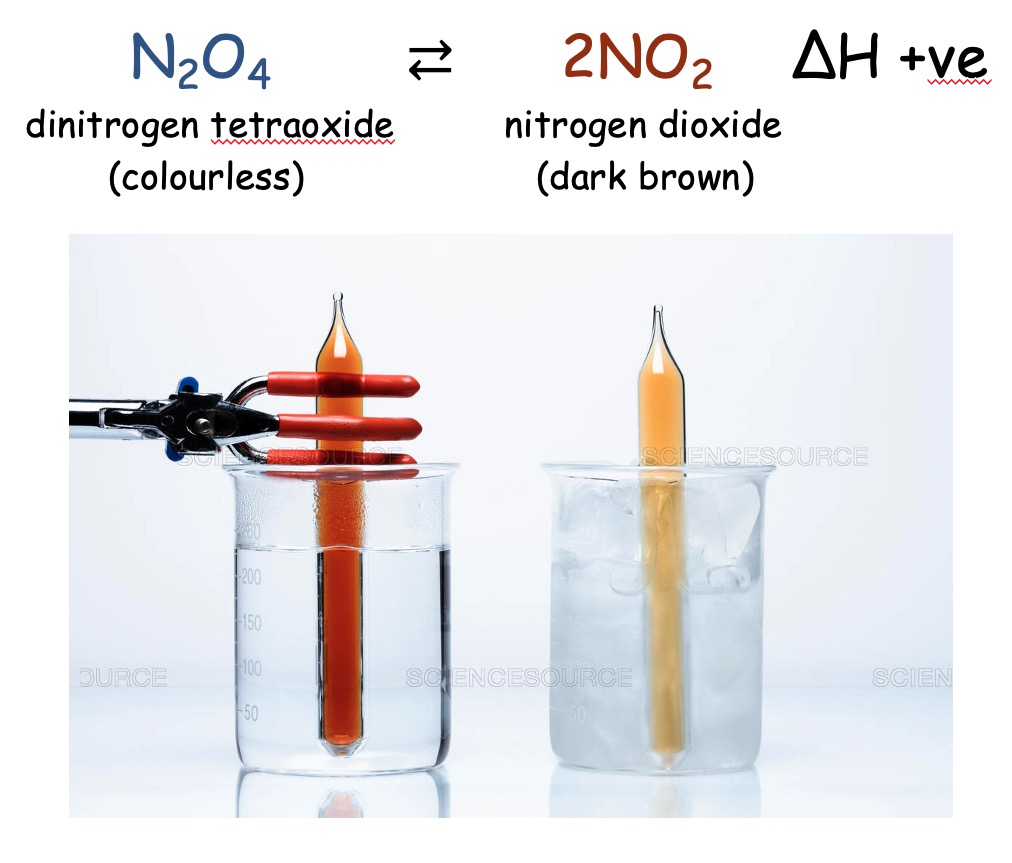
Increasing the temperature favours the forward reaction as it takes in energy, so the equilibrium moves to the right producing more NO2 and less N2O4. So the colour becomes darker.
Decreasing the favours the reverse reaction as it gives out energy so the equilibrium moves to the left producing more N2O4 and less NO2. So the colour becomes lighter.
Consider the follwing Equilibrium:
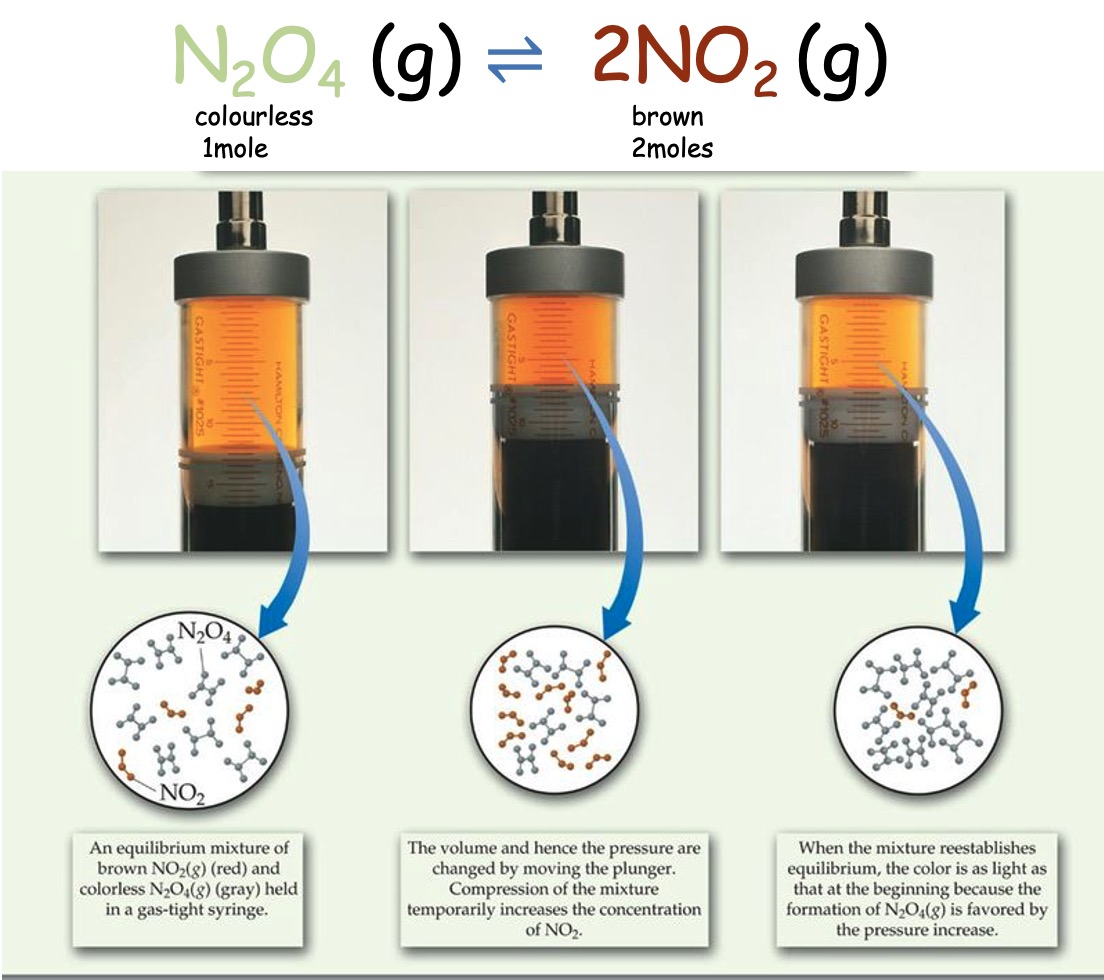

Increasing the pressure will cause the equilibrium to move to decrease the pressure. The equilibrium will move to reduce the number of gas particles.
The equilibrium moves to the left producing more N2O4 and less NO2 so the colour lightens.
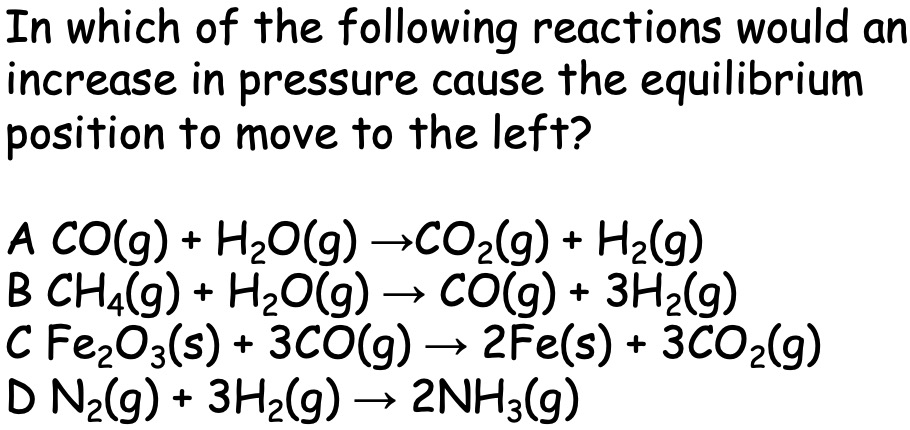
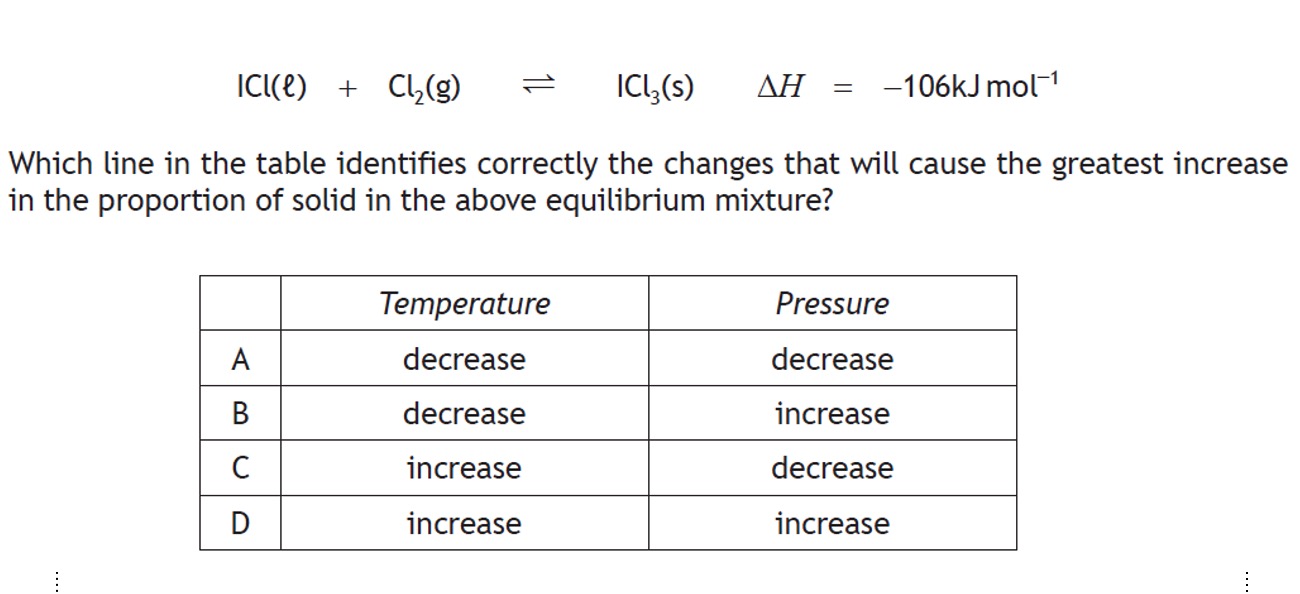
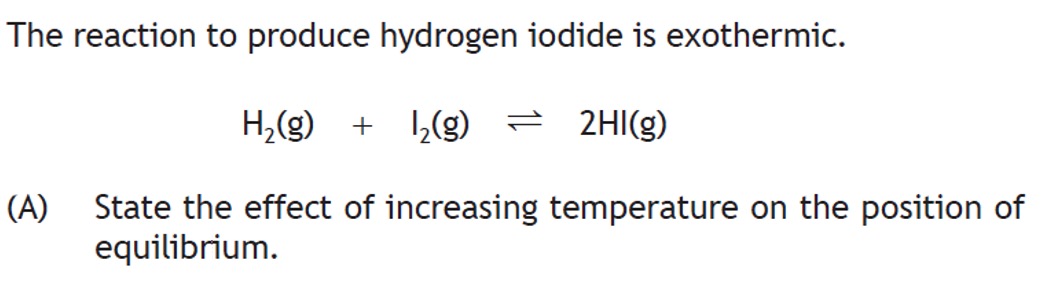

Consider the follwing Equilibrium:
An increase in concentration of A or B will speed up the forward reaction, thus increasing the concentration of C and D.
A similar effect can be achieved by reducing the concentration of C or D.
A mixture of cobalt chloride and conc HCl sets up the following equilibrium:
Co(H2O)62+ + 4Cl- ⇋ CoCl42- + 6H2O ΔH +ve
Adding extra Cl- ions forces the equilibrium to try to remove these. The forward reaction is favoured because this uses up chloride ions.
The equilibrium has moved to the right so the solution becomes blue in colour.
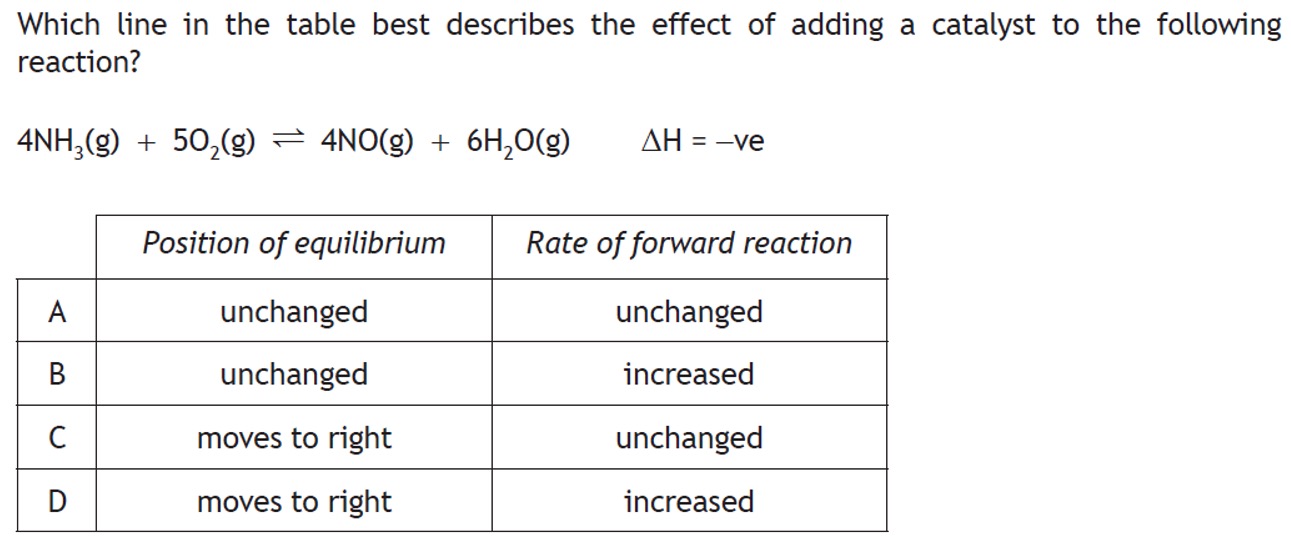
The addition of a catalyst increases the rates of the forward and reverse reactions equally. The catalyst increases the rate at which equilibrium is achieved but does not affect the position of equilibrium.

Dissolving chlorine in water produces the hypochlorite ion, ClO-, which has a bleaching effect.
Cl2 + H2O ⇋ 2H+ + ClO- + Cl-

Adding an alkali will remove hydrogen ions from the equilibrium which will move to the right to replace them.
The bleaching effect will be increased.
Adding silver nitrate will remove chloride ions from the equilibrium as the precipitate silver nitrate is formed. (check p21 of the databook for solubilities).
The equilibrium will move to the right to replace them so the bleaching effect will be increased.
Adding an acid causes the equilibrium to move to 'use up' H+ ions. The equilibrium moves to the left producing more toxic Cl2.
This can be fatal and accidents caused by mixing bleach and acid are not unusual.