

The enthalpy of combustion is the heat energy given out when 1 mole of fuel burns completely in oxygen.
The enthalpy of combustion of methanol can be represented by the equation:
CH4(g) + O2(g) → CO2(g) + H2O(l)
The heat energy released when alcohols burn can be measured.
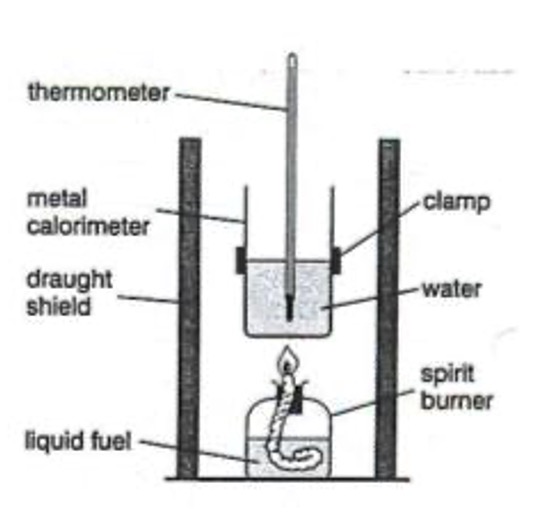
The enthalpy of combustion of a substance is the amount of energy given out when one mole of a substance burns in excess oxygen.
Suppose 0.25 g of ethanol had been burned to raise the temperature of 100g of water 12.5 oC.
Step 1 - The heat energy gained by the water (Eh) is calculated using the formula:
Eh = cmΔT
Eh = 4.18 x 0.10 x 12.5
Eh = 5.225 kJ
The heat energy released on burning 0.25 g of ethanol = 5.225 kJ
Step 2 - The mass of one mole of ethanol can be calculated:
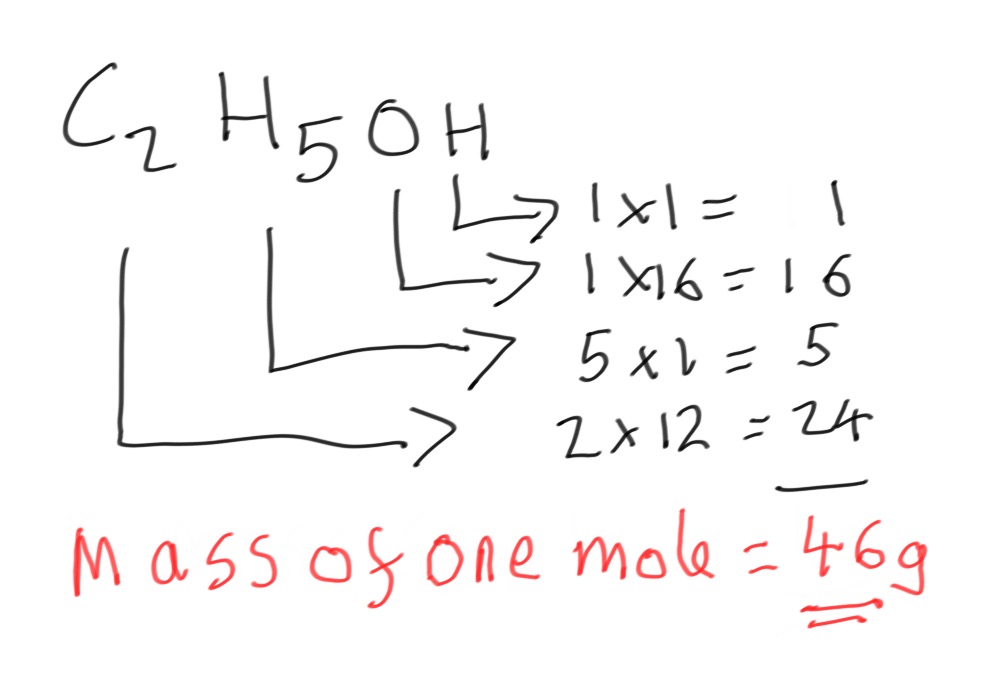
Step 3 - The heat energy released on burning 1 mole of ethanol can now be calculated:
ΔH =( Eh x n ) / m
ΔH = (5.225 x 46) / 0.25
ΔH = 961KJ
The enthalpy of combustion of ethanol = - 961 kJ mol-1
(A negative sign is used because combustion is an exothermic reaction)