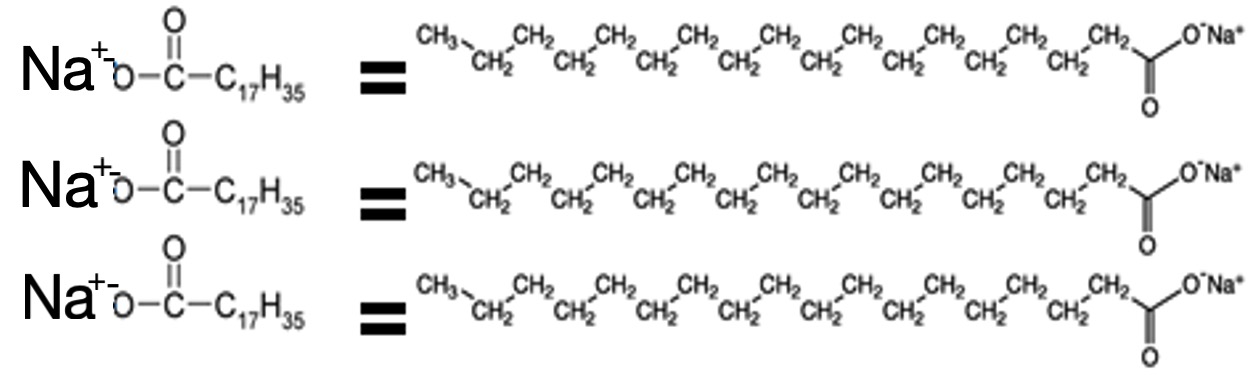
 Soaps are salts of fatty acids.
Soaps are salts of fatty acids.
Alkaline hydrolysis is used to make sodium salts of fatty acids.
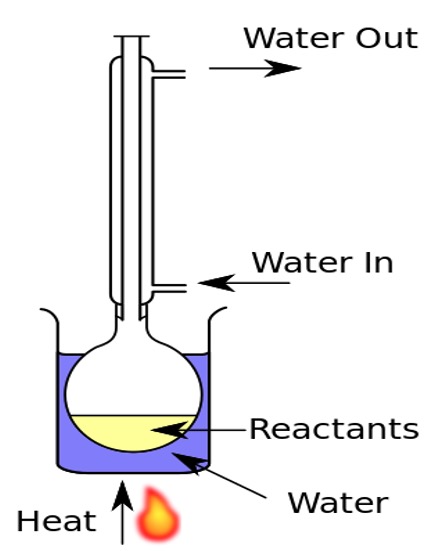
Soaps are formed by the alkaline hydrolysis of fats and oils by sodium or potassium hydroxide by boiling under reflux conditions.
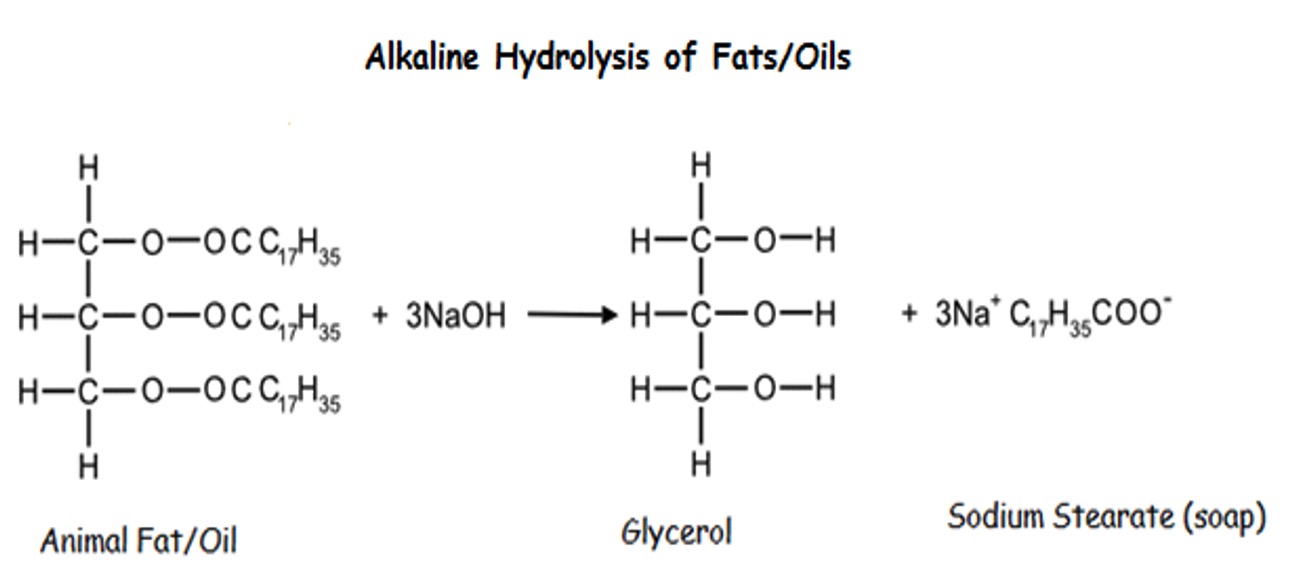
Glycerol is produced as a by-product of the hydrolysis of fat.
It is separated and used as a raw material for other processes and preparations for example antifreeze.
It can also be used to produce trinitroglycerin, which is an essential ingredient of various explosives such as dynamite.
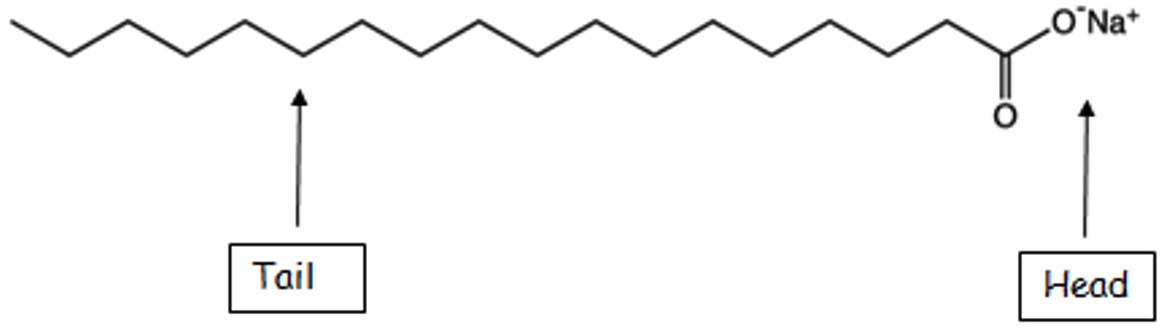
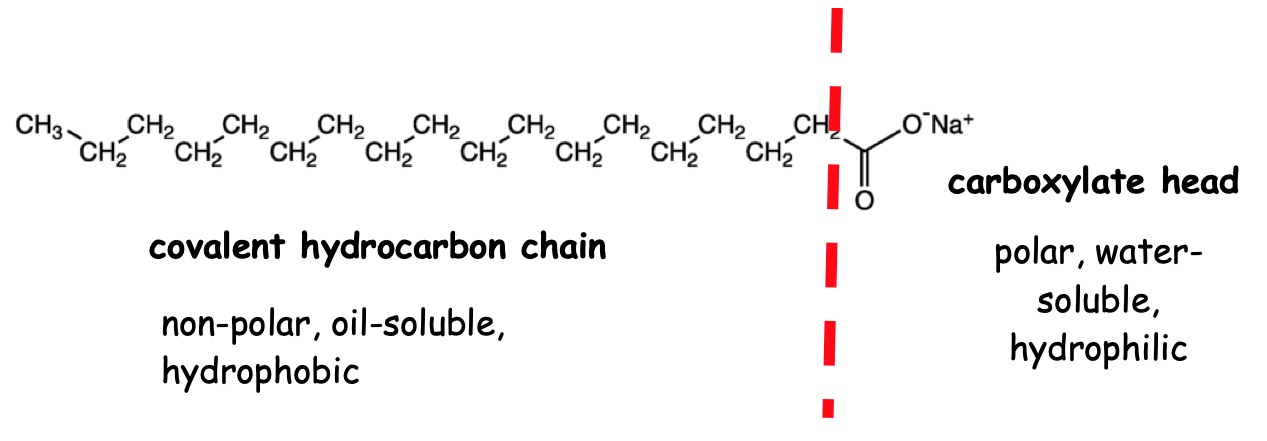
The tail of a soap is made of a long covalent hydrocarbon chain.
The charged carboxylate group represents the head section of the soap structure.
Why do we need soaps?
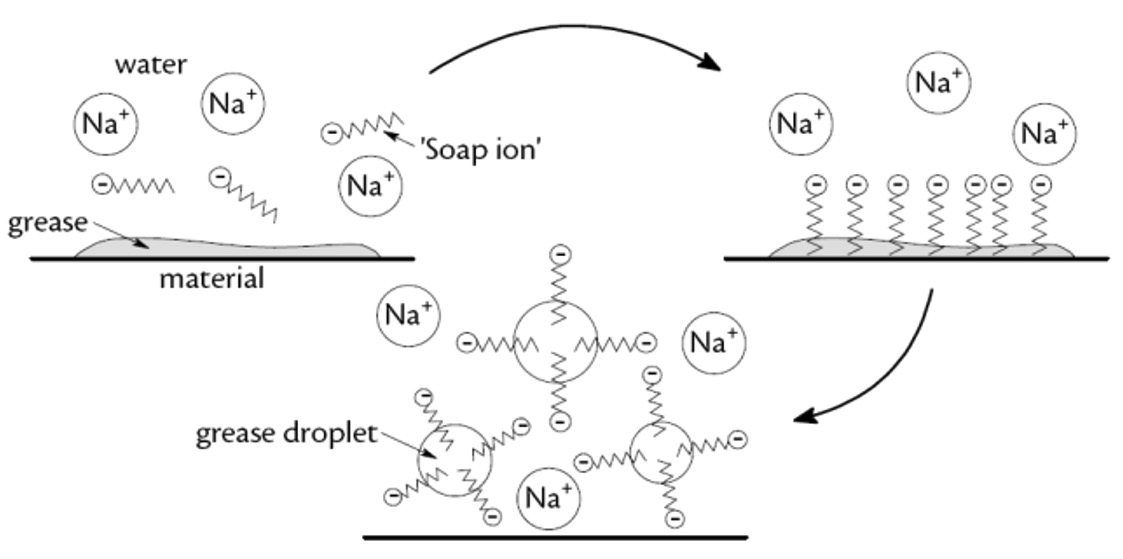
Cleaning with water alone has little effect when stains consist of non-polar substances, such as grease and sweat since fats do not dissolve in water.
This is because water is a polar solvent but fats and oils are non-polar.
The long covalent hydrocarbon chain gives rise to the hydrophobic (water hating) and oil-soluble (non-polar) properties of the soap molecule.
The charged carboxylate group is attracted to water molecules (hydrophilic – water loving).
In solution a soap molecule consists of a long non-polar hydrocarbon tail (C17H35-) and a polar head (-COO-).
The non-polar hydrophobic tail is soluble in non-polar substances such as oil, while the hydrophillic polar head is soluble in polar substances, such as water.
Agitation produces miscelles which suspends the oil droplets in the water.
The negatively-charged heads repel like charges on other oil droplets, so the oil layer does not reform.
This represents the initial interaction of soap on addition to water and material with a grease stain. This repulsion prevents the oil droplets re-joining and helps disperse the oil.
When used for cleaning in combination with water, soap serves as a surfactant. Surfactants are the main contributors to detergents' cleaning power.
A broad definition of a surfactant is:
a substance, such as soap, that possesses a hydrophobic tail and a hydrophilic head and which, on being made into a solution with water, reduces the surface tension of water and also reduces the interfacial tension between oil and water.
The bulk components of detergents are surfactants; other key ingredients include:
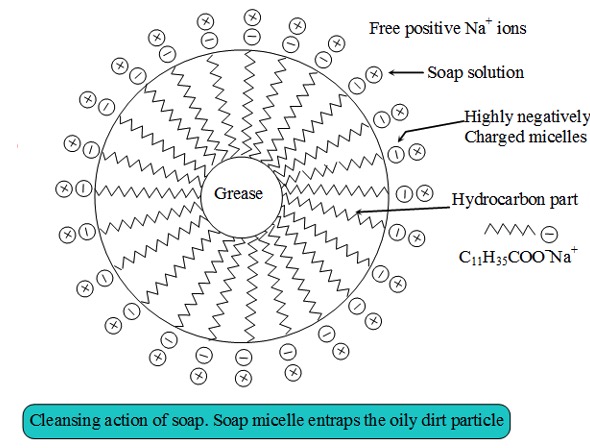
The hydrophobic tails 'burrow' into the droplet of oil or grease. The hydrophilic heads are left to face the surrounding water.
This results in the formation of a ball-like structure (a micelle).
The non-polar substances, such as oil or grease, are held inside the ball and suspended in water, to be washed away.
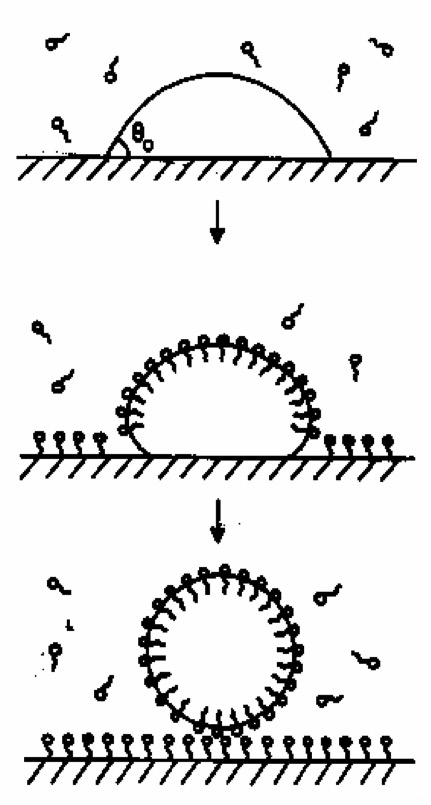
Soapless detergents are substances with non-polar hydrophobic tails and ionic hydrophilic heads.
These remove oil and grease in the same way as soap. Soapless detergents do not form scum with hard water.
Hard water is a term used to describe water containing high levels of dissolved metal ions. When soap is used in hard water, scum, an insoluble precipitate, is formed.
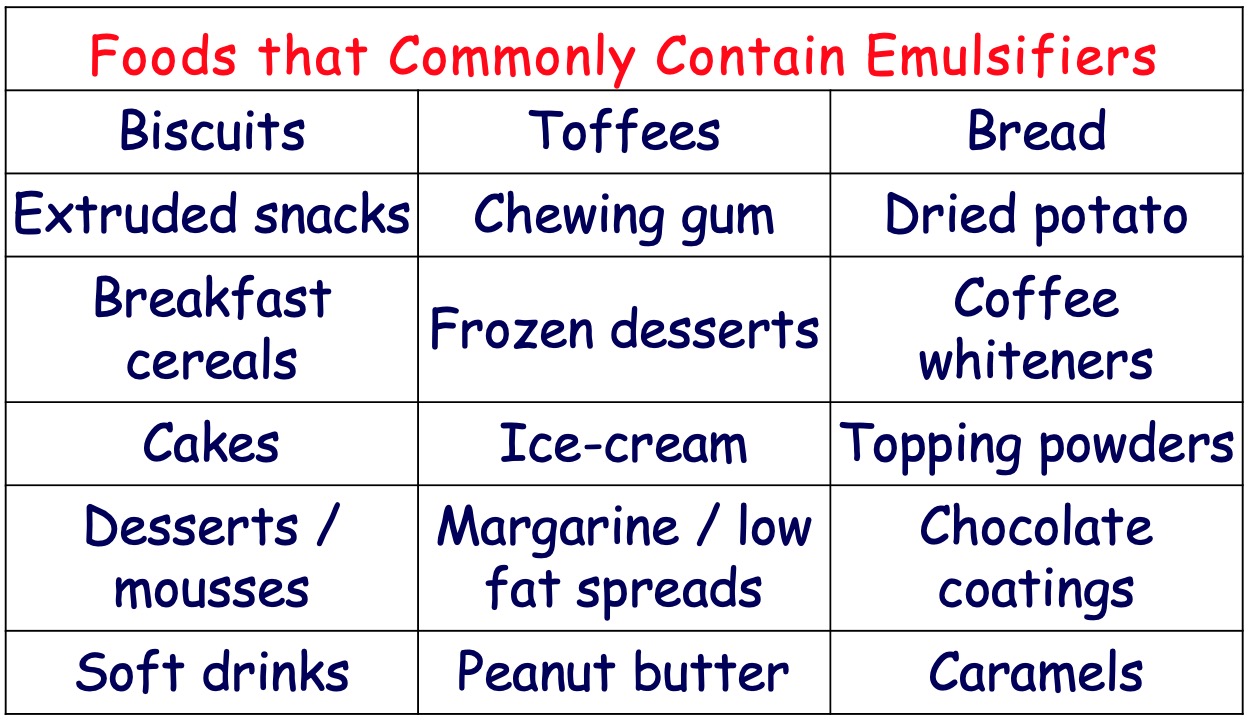
An emulsion contains small droplets of one liquid dispersed in another liquid.
Emulsions in food are mixtures of oil and water. To prevent oil and water components separating into layers, a soap-like molecule known as an emulsifier is added.
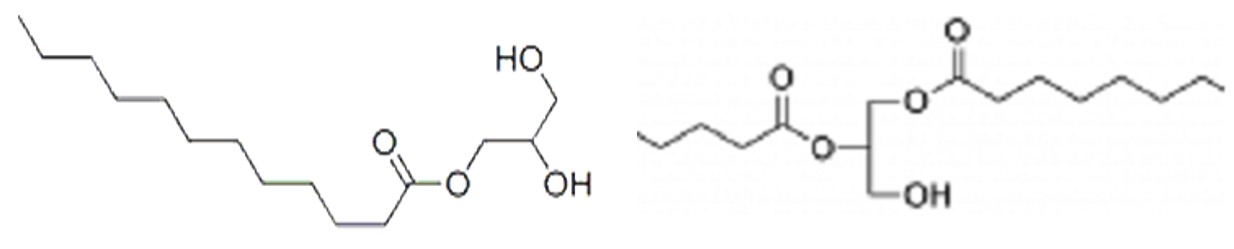
Emulsifiers for use in food are commonly made by reacting edible oils with glycerol to form molecules in which either one or two fatty acid groups are linked to a glycerol backbone rather than the three normally found in edible oils.
The one or two hydroxyl groups present in these molecules are hydrophilic whilst the fatty acid chains are hydrophobic.
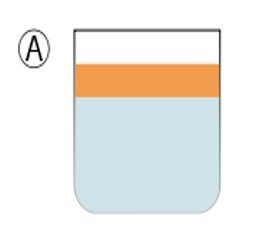 Two liquids not yet emulsified form two separate phases, a layer of oil on top of a layer of water.
Two liquids not yet emulsified form two separate phases, a layer of oil on top of a layer of water.
 The liquids have been agitated (stirred vigorously), initially the water layer and oil layers have formed an emulsion.
The liquids have been agitated (stirred vigorously), initially the water layer and oil layers have formed an emulsion.
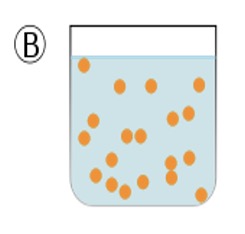
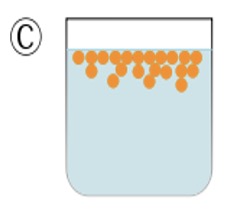 In diagram C the unstable emulsion progressively separates back into two distinct layers (phases).
In diagram C the unstable emulsion progressively separates back into two distinct layers (phases).
Diagram C shows the result after a few minutes, the two liquids return to form two separate phases, a layer of oil on top of a layer of water.
However some substances are able to stabilise an emulsion and prevent two separate layers forming again.
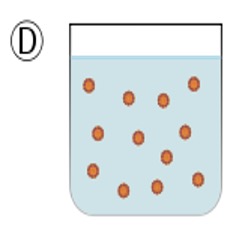 This shows the addition of an emulsifier. This allows the two immiscible layers to be mixed uniformly.
This shows the addition of an emulsifier. This allows the two immiscible layers to be mixed uniformly.
 The presence of this emulsifier is shown on packaging by E-numbers, E471 and is one of the most common on food packaging.
The presence of this emulsifier is shown on packaging by E-numbers, E471 and is one of the most common on food packaging.
Mayonnaise contains oil and water. The emulsifier keeps these mixed and without it the oil and water separate.