

Alkanes are a group of hydrocarbons that are made up of chains of carbon atoms joined together by single covalent bonds.
Each carbon atom has the maximum number of hydrogen atoms that can bond to it and they are often described as being saturated and insoluble in water.
Alkanes have a general formula given by:
CnH2n+2
where n = the number of carbon atoms given by the prefix.
e.g. propane:
Full Structural Formula:
Shortened Structural Formula:
Chemical or Molecular Formula:
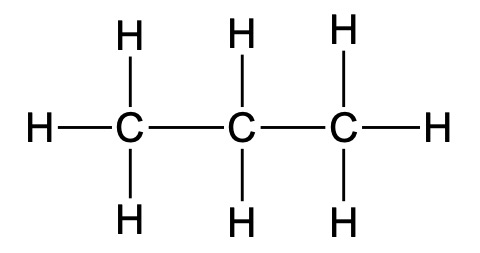
CH3CH2CH3
C3H8
Alkenes are made up of chains of carbon atoms joined together by covalent bonds. At least one of the bonds must be a double bond.
Alkenes are often described as being unsaturated and insoluble in water.
Alkenes have a general formula given by:
CnH2n
where n = the number of carbon atoms given by the prefix.
e.g. propene
Full Structural Formula:
Shortened Structural Formula:
Chemical or Molecular Formula:
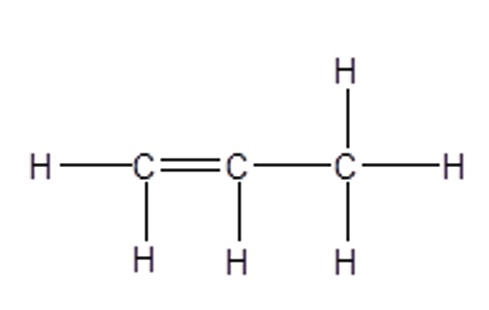
CH2=CHCH3
C3H6
Cycloalkanes are another type of hydrocarbon that can be formed by a variety of chemical reactions in the petrochemical industry.
Like alkanes they are described as being saturated as they contain only single bonds between the carbon atoms, but unlike alkanes they form a ring structure. They are also insoluble in water.
Cycloalkanes have a general formula given by:
CnH2n
where n = the number of carbon atoms given by the prefix.
e.g. cyclopropane
Full Structural Formula:
Shortened Structural Formula:
Chemical or Molecular Formula:
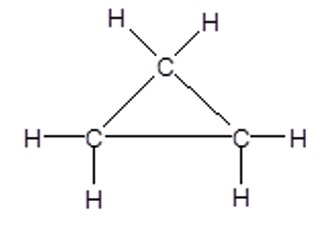
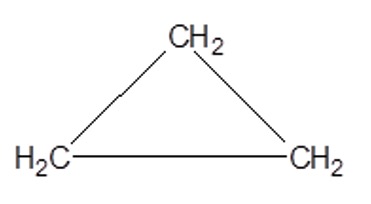
C3H6
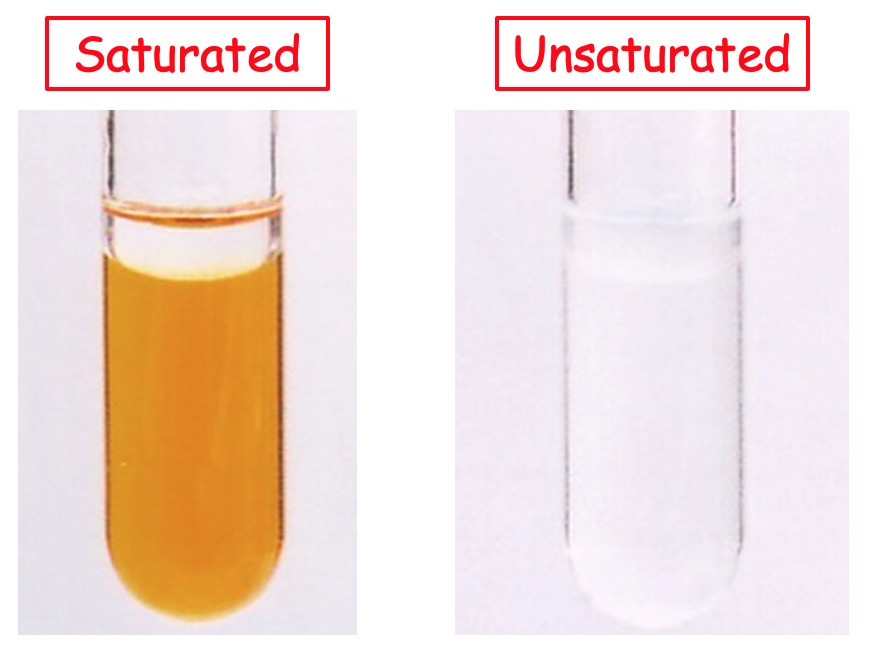
Unsaturated molecules such as alkenes can be identified using bromine water.
Bromine water will be decolourised in the presence of alkenes
Other small molecules can react with alkenes in similar ways.
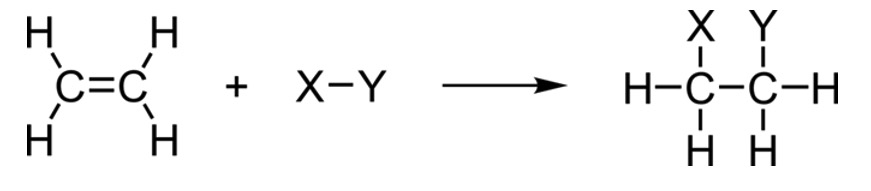
Hydrogenation (H2)
Alkenes become alkanes with the addition of hydrogen. This is called hydrogenation.
Hydration (H2O)
Alkenes become alcohols with the addition of water. This is called hydration and is a reversible reaction.
Isomers are compounds which have the same molecular formula but different structural formulae.
Systematic Naming Rules