Esters are compounds formed by a condensation reaction between alcohols and carboxylic acids.
In a condensation reaction two molecules join and a small molecule (often water) is removed.
The reaction can also be referred to as esterification.
General Word Equation:
Alcohol + Carboxylic Acid → Ester + Water
Generally (but not always) the acid part of the ester is drawn first and the alkanol part second. For example, propyl butanoate:
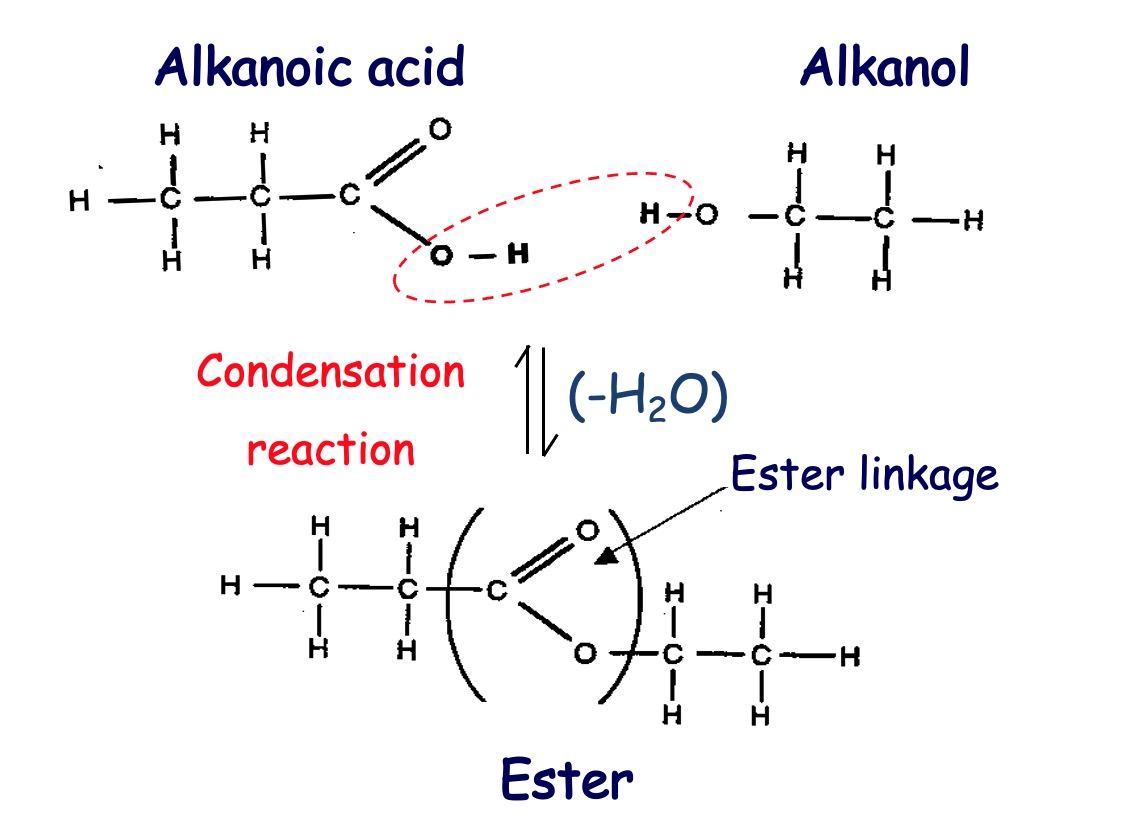
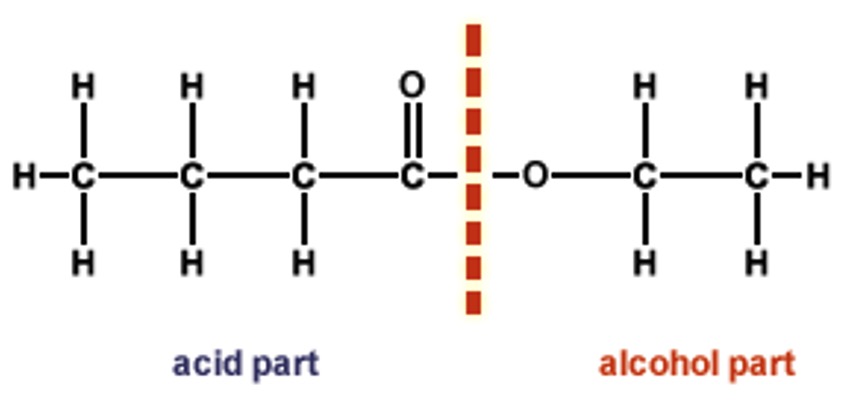
The OH from the acid and the H from the alcohol form water.
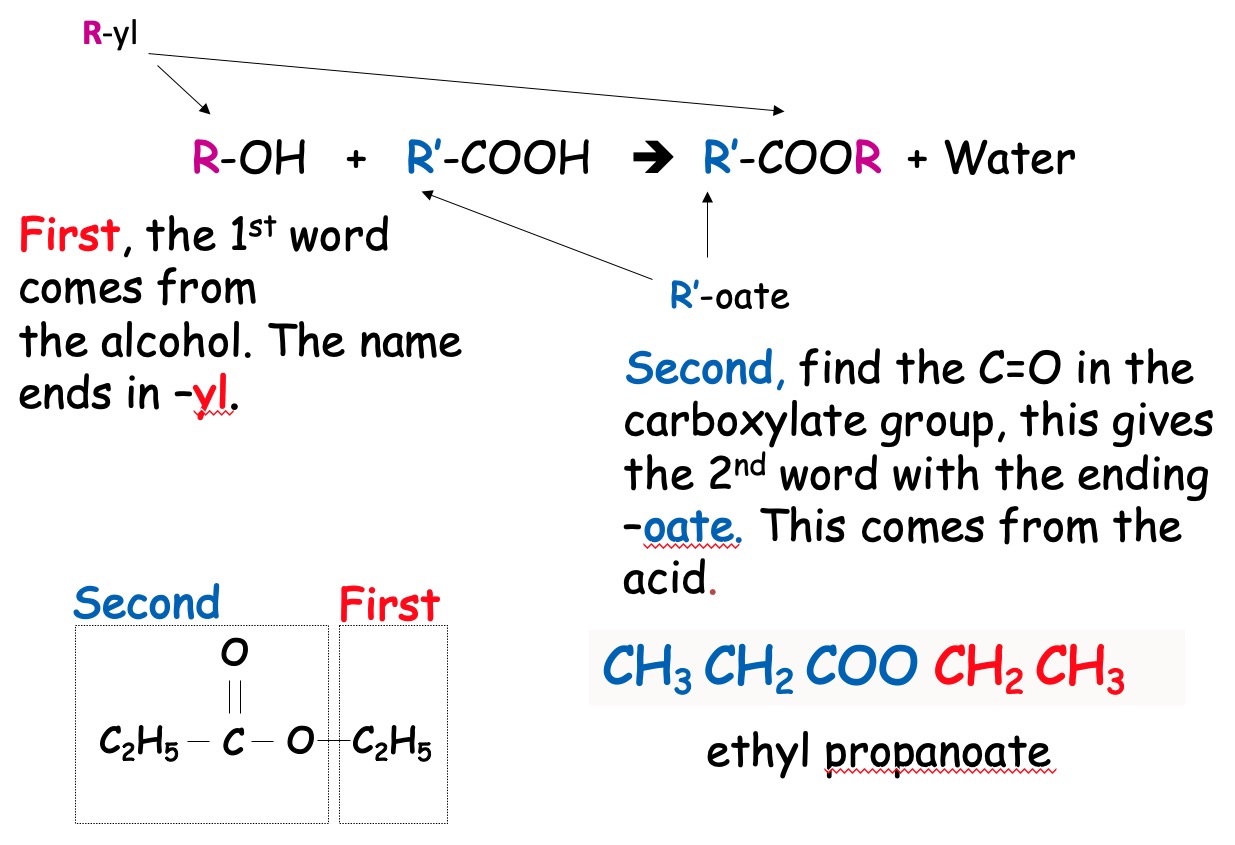
The alkanol part of the ester is named first. The “anol” ending is replaced by “yl”
e.g.
Propanol → propyl
Methanol → methyl
The acid part of the ester is named second. The “oic” acid is replaced by “oate”
e.g.
Butanoic acid → butanoate
Ethanoic acid → ethanoate
propanol + butanoic acid → propyl butanoate
methanol + ethanoic acid → methyl ethanoate
e.g.
One way of preparing esters is to condense an alcohol with a carboxylic acid:
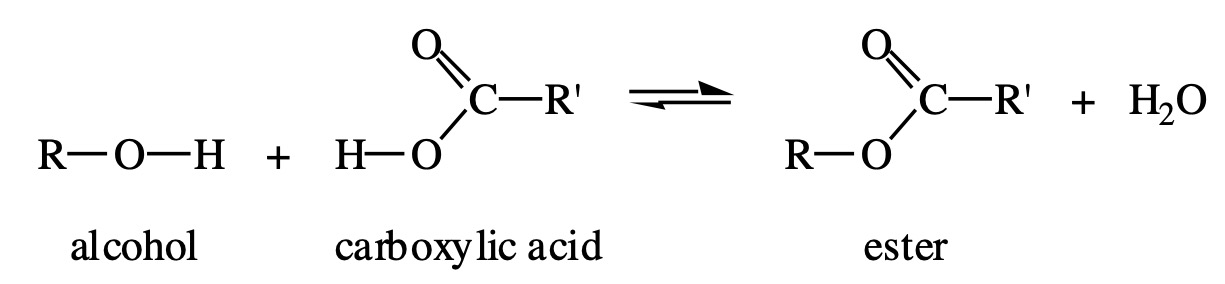
The reaction is slow at room temperature and the yield of ester is low. The rate can be increased by heating the reaction mixture and by using concentrated sulphuric acid as a catalyst. The presence of the concentrated sulphuric acid also increases the yield of ester.
The aim of this experiment is to prepare an ester and to identify some of the characteristic properties of esters.
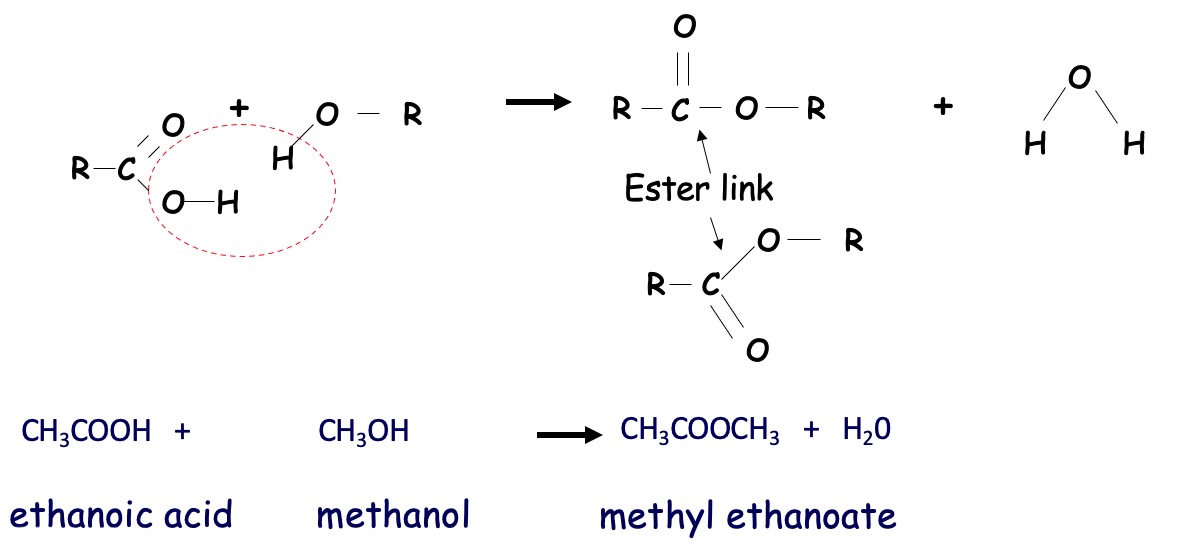
The reaction is brought about by heating a mixture of a carboxylic acid and an alcohol with a little concentrated sulphuric acid. (which acts as a catalyst and absorbs the water produced).
Esters can be used as solvents in car body paints, radiator enamels, adhesives and cosmetic preparations such as nail varnish.
This is due to the fact that the smaller esters, like ethyl ethanoate, are very volatile and therefore evaporate very quickly.
Ethyl ethanoate is one of a number of solvents used to extract caffeine from coffee and tea.
De-caffeinated products produced with ethyl ethanoate are often described on the packaging as "naturally decaffeinated" because ethyl ethanoate is a chemical found naturally in many fruits.
They also have medicinal uses such as aspirin.
In hydrolysis reactions large molecules are broken down into smaller molecules by water. Esters can be hydrolysed to produce the parent alkanoic acid and the parent alkanol.
The formation of esters by the condensation is a reversible reaction. This means that not only can the reactants form products but the products can change back into reactants.
Hydrolysis is the process by which water breaks down a carbon compound, in practice, water on its own is rarely successful in achieving this.
Hydrolysis of an ester is usually carried out by heating it in the presence of a dilute acid, such as HCl or H2SO4, to provide hydrogen ions to catalyse the hydrolysis.
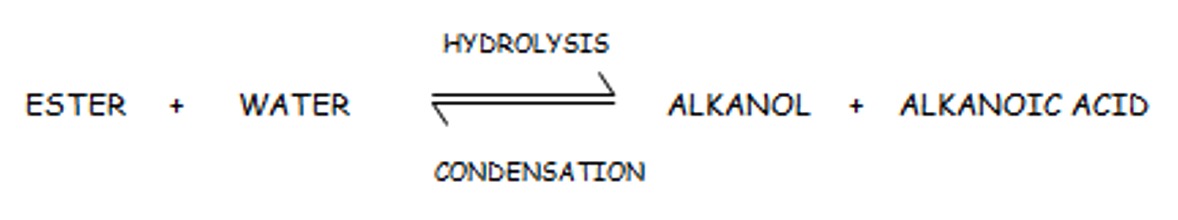
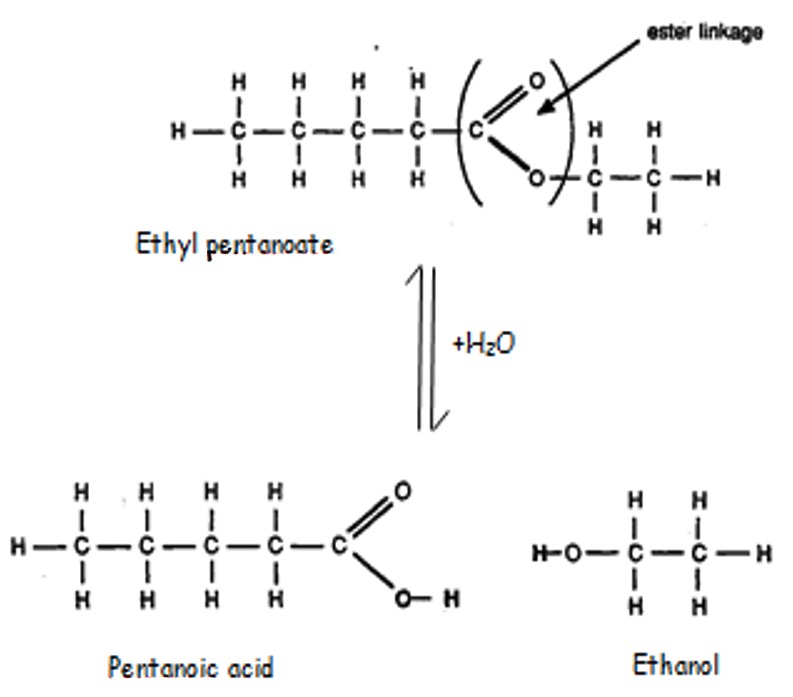
Note: during the hydrolysis water breaks down the ester by breaking the ester linkage. The -OH adds back on to the acid and the H adds back on to the alkanol.
Fats and oils are an essential part of the human diet. They provide energy for movement, growth and repair.
Fats and oils are a more concentrated source of energy than carbohydrates.
Fats and oils help humans feel fuller for longer due to their high satiety level.
Fat molecules are insoluble, tend to group together and form a large droplet stored in tissue. We store our extra energy as fat.
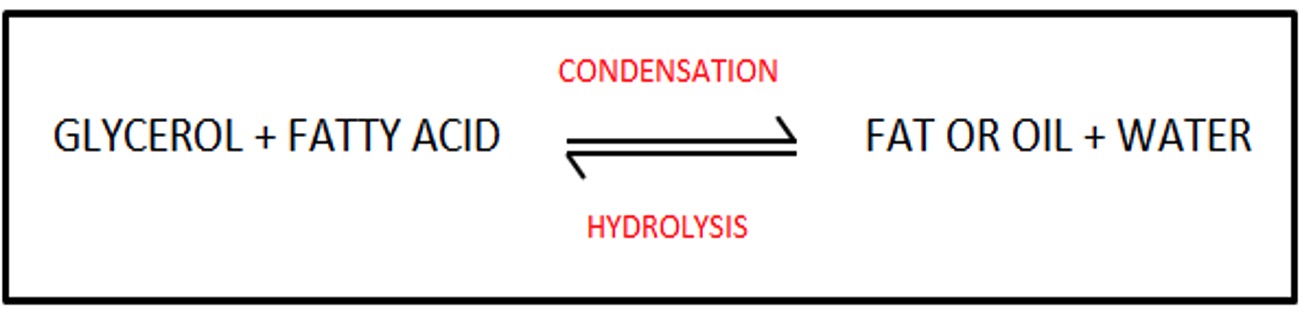
Edible fats and edible oils are naturally occurring esters of the alcohol, glycerol and long chain alkanoic acids.
Fats and oils are formed by the condensation reaction of the two reactants.

Glycerol (propane-1,2,3-triol) is a trihydric alkanol as contains three hydroxyl groups.
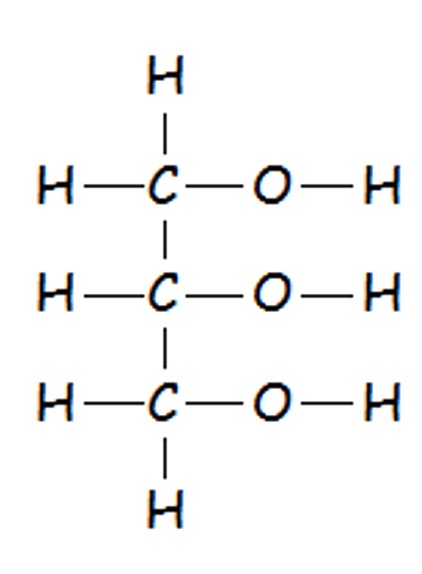
Fats and oils are actually special forms of esters where the alcohol, glycerol (propane-1,2,3-triol) has three hydroxyl groups.

Glycerol is termed a 'trihydric alcohol'.
Glycerol can therefore make three ester links when reacting with three long carboxylic acids (fatty acids.)
1 glycerol reacts with 3 acids
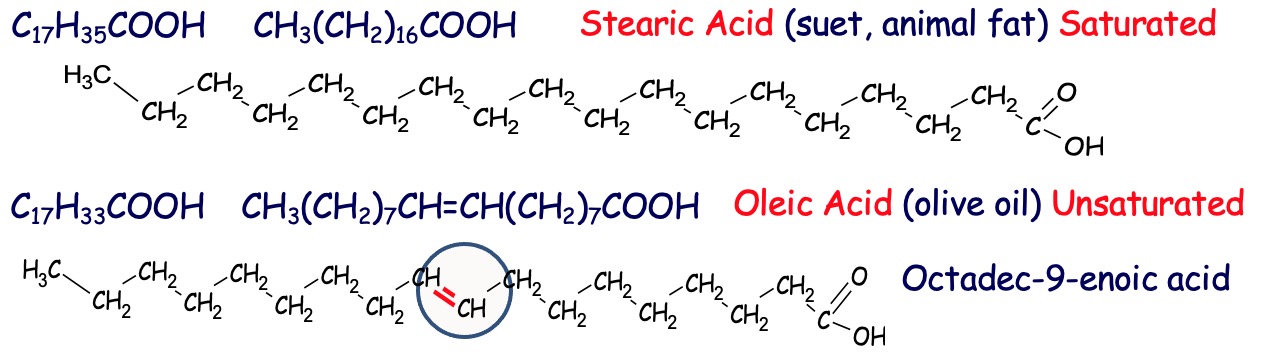
The long chain alkanoic acids are often referred to as fatty acids.
The hydrocarbon chain in each can be from 4 to 24 C's long
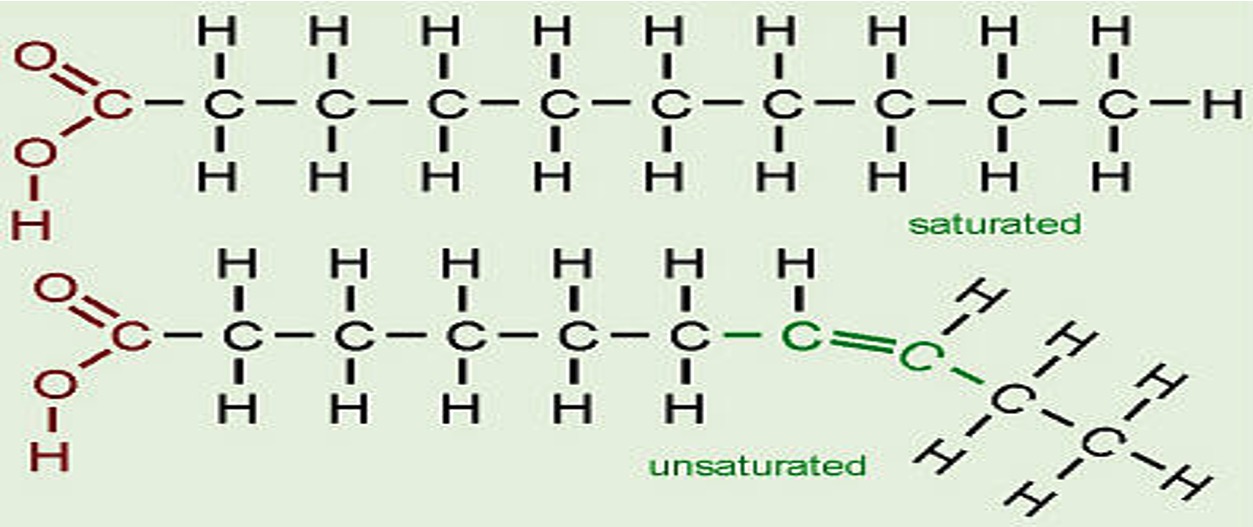
The C's can be single bonded (saturated) or double bonded (unsaturated).
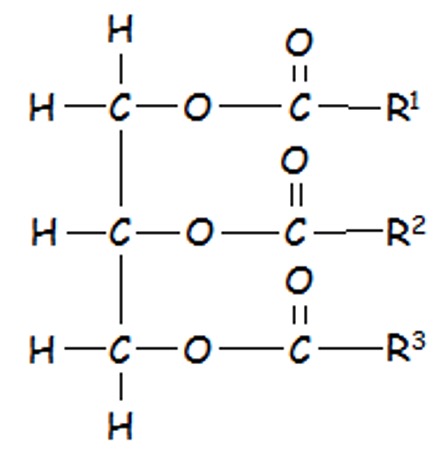
Fats and Oils are formed by combination of 3 moles of fatty acids to 1 mole of glycerol - a triglyceride. There are 3 ester linkages in the one molecule and 3 moles of water are removed.
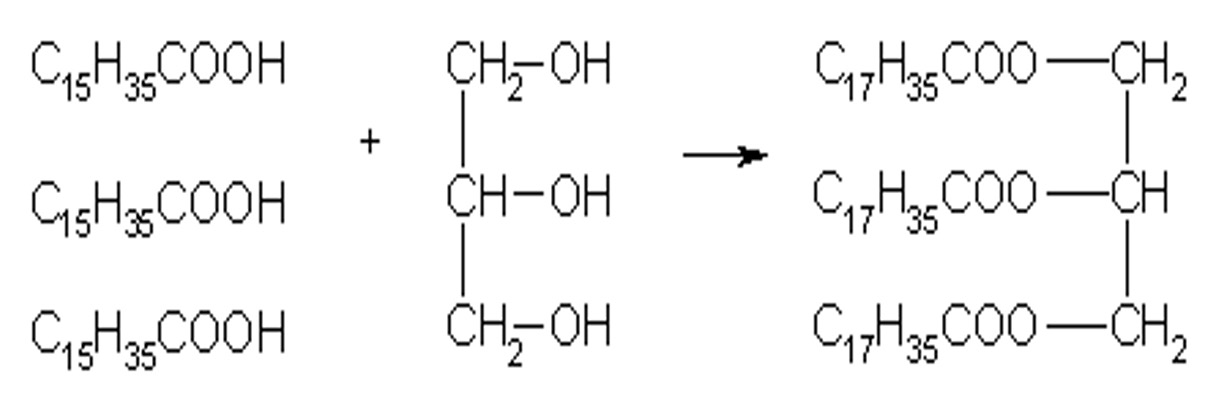
Although fats and oils are formed by the same process and from the same reactants, fats and oils have different degrees of unsaturation.
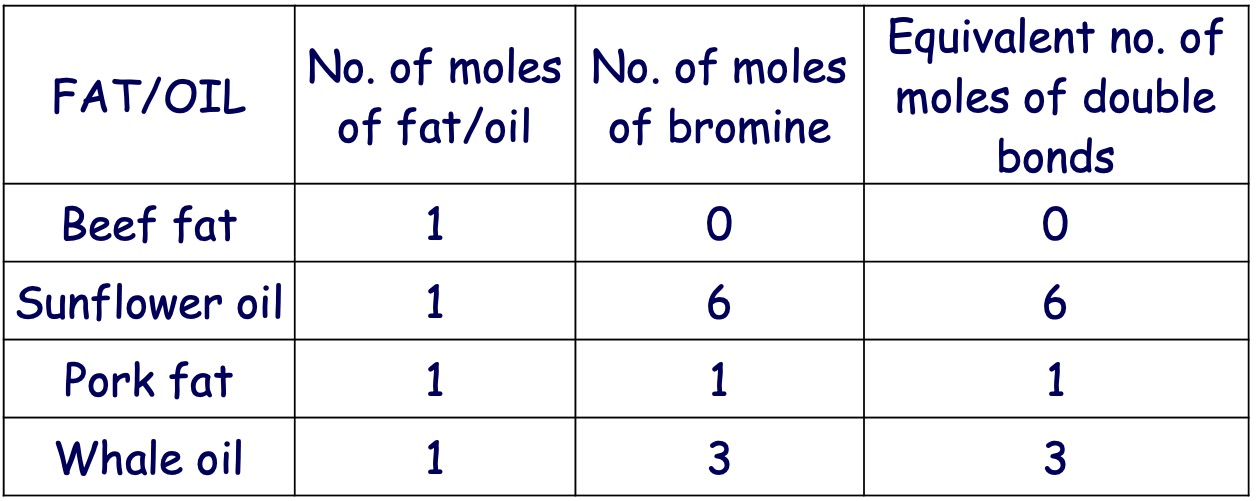
When oil is shaken with bromine water, the brown colour of bromine water is lost rapidly.
This indicates the presence of double bonds in the oil molecules.
If a fat is dissolved in an organic solvent such as hexane and then shaken with bromine water, no decolourisation occurs with some fats and only slight or slow decolourisation with others.
This indicates that fats may contain no double bonds or fewer double bonds than oils.
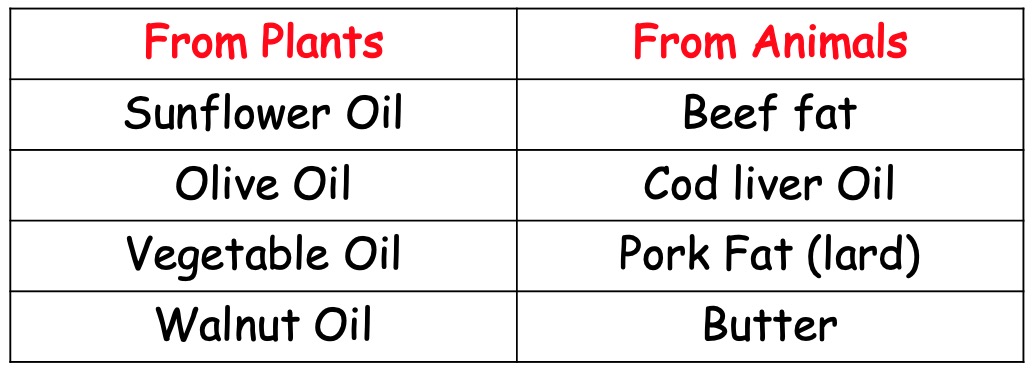
The main difference between fats and oils is that;
This is because oils have a lower melting point than their fat counterparts due to the greater amount of unsaturation within the (oil) molecules.
You can titrate fats/oils against bromine water and to determine the volume (and hence the number of moles) of bromine needed to react with all of the double bonds in 1 mole of the fat/oil molecules.
This will establish the degree of unsaturation
The greater the number of drops, the more unsaturated the oil.
Since glycerol is constant, it is in the acid chain that we potentially find the double bond - if there is a double bond the acid is called an alkenoic acid.
Fatty acids are saturated or unsaturated straight chain carboxylic acids with even numbers of C atoms ranging from C4 to C24, but mainly C16 to C18.
Bromine can be used to distinguish between saturated and unsaturated molecules. (unsaturated fats/oils will decolourise bromine).
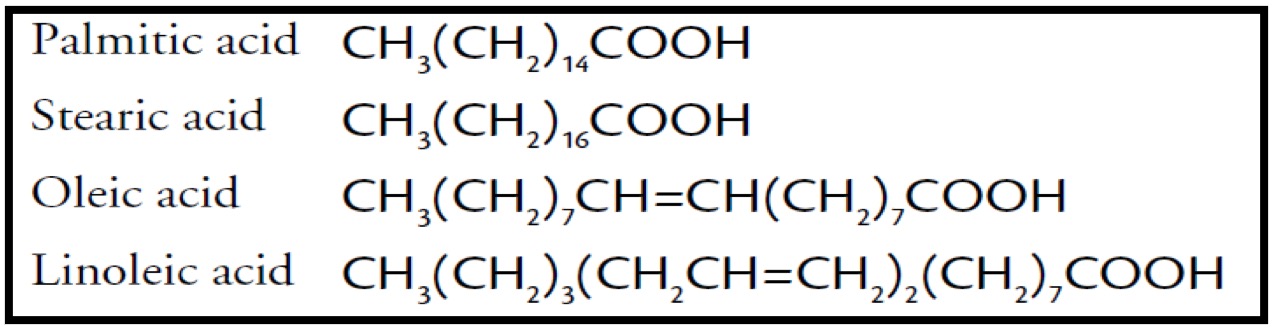
Each double bond will react with 1 molecule of bromine or each mole of double bonds will react with 1 mole of bromine.
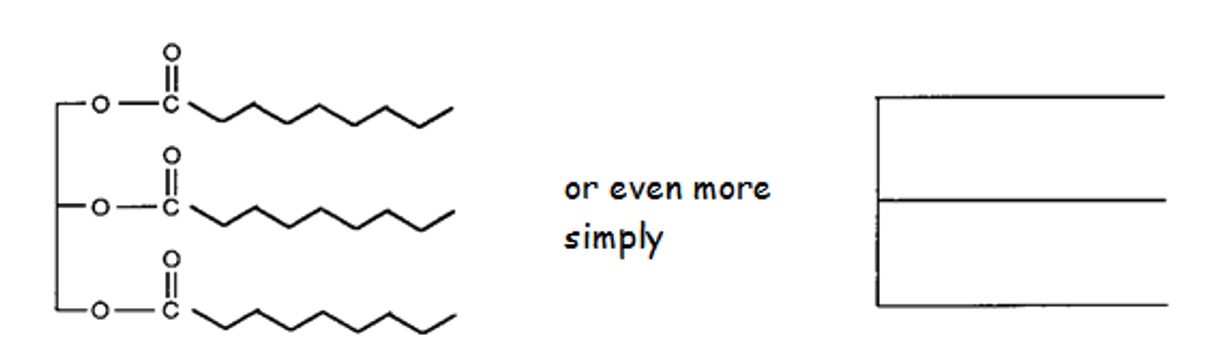
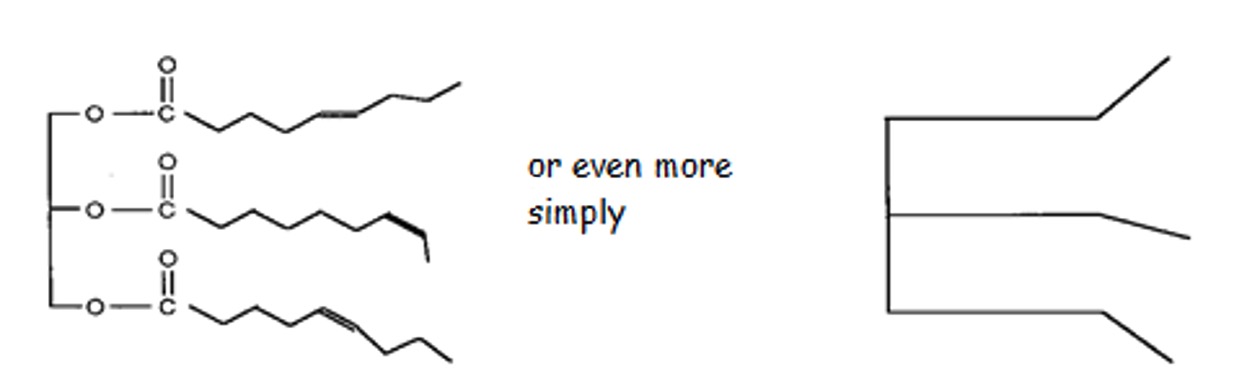
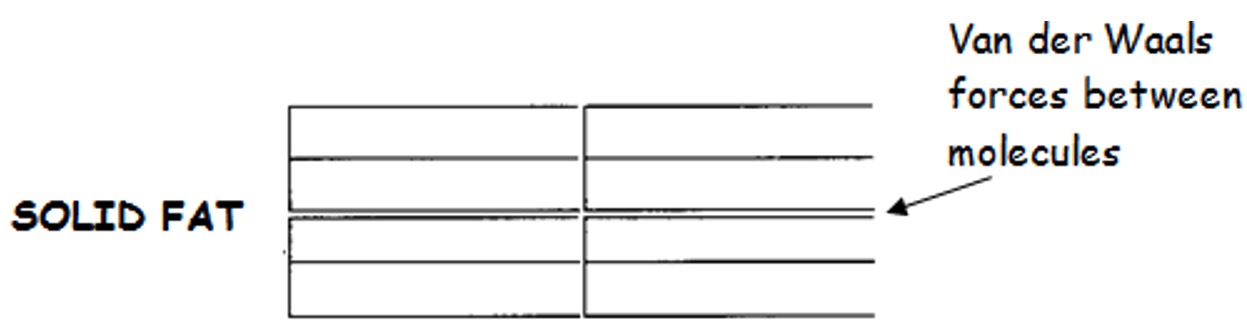
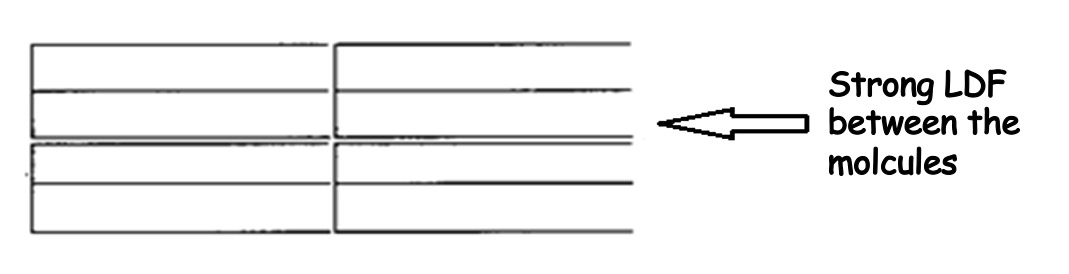
This means that these molecules will be able to stack closely together and as a result they will have strong Van der Waals forces and relatively high melting points.
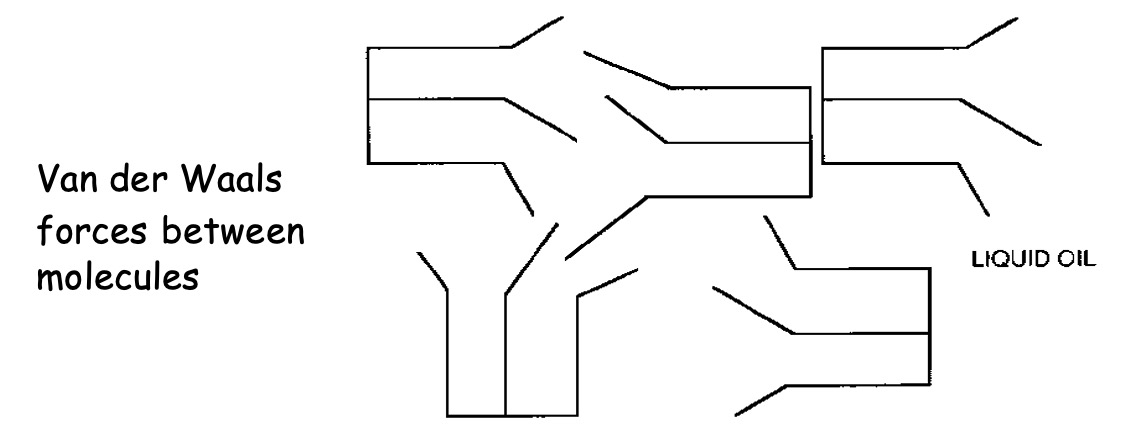
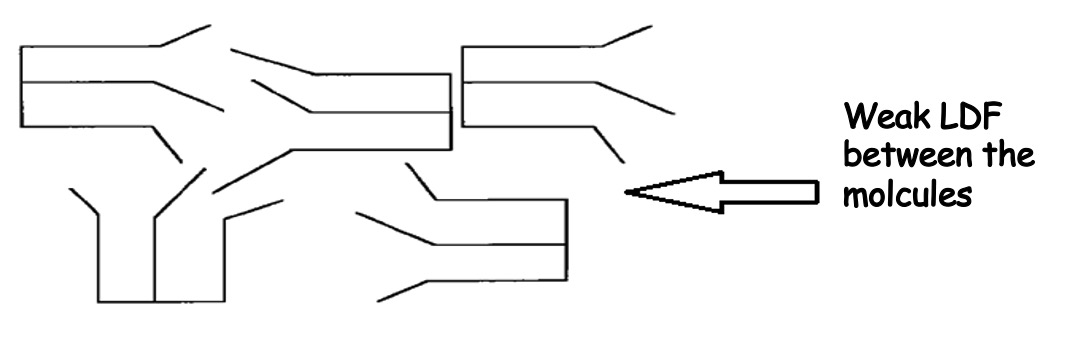
The double bonds in the unsaturated fatty acids cause "kinks" in the chain. As a result these molecules cannot pack as closely together hence the Van der Waals' forces are not as strong so oils tend to have low melting points than fats.
The absence of a double bond allows the fat molecules to be more regularly 'tuning fork' shaped and consequently the fat molecules can fit into one another.
If a double bond is present then the oil (and some fats) molecules zigzag and the molecule chains become distorted and cannot fit into one another.
Molecules which can pack closely together due to their regular structure have stronger London Dispersion forces between the molecules and thus higher melting points.
Therefore fats have higher melting points than oils - and fats are solid at room temperature.
Arrangement of Fat Molecules
Arrangements of Oil Molecules
Fats and oils are made by a condensation reaction between glycerol and fatty acids.
Fats and oils can therefore be hydrolysed to produce these molecules.
Hydrolysis of fat or oil is similar to that of an ester.
The ester link breaks due to the addition of water and one molecule of glycerol is produced along with 3 fatty acid molecules.
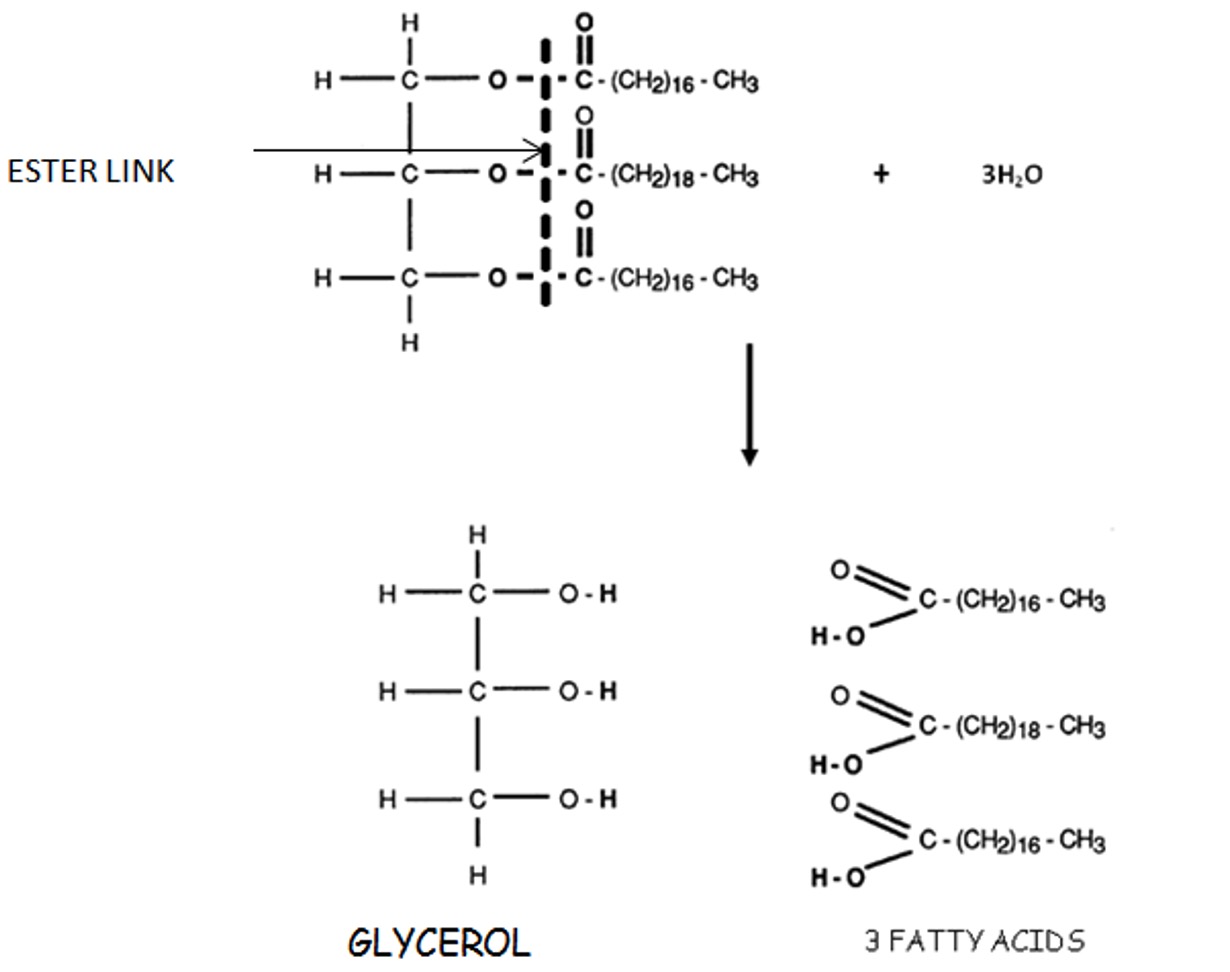
The addition of water splits the fat or oil in to 1 molecule of glycerol and 3 molecules of fatty acids.