

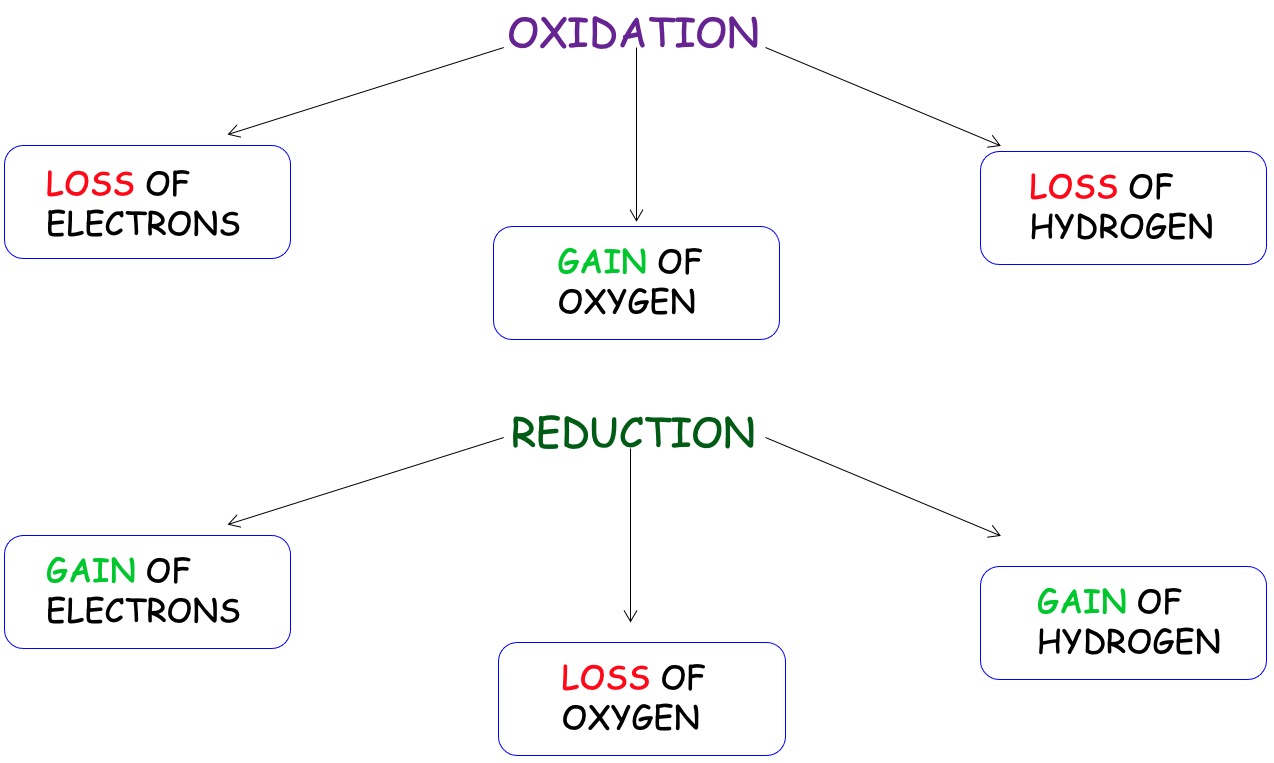
A redox reaction involves two half reactions: oxidation and reduction.
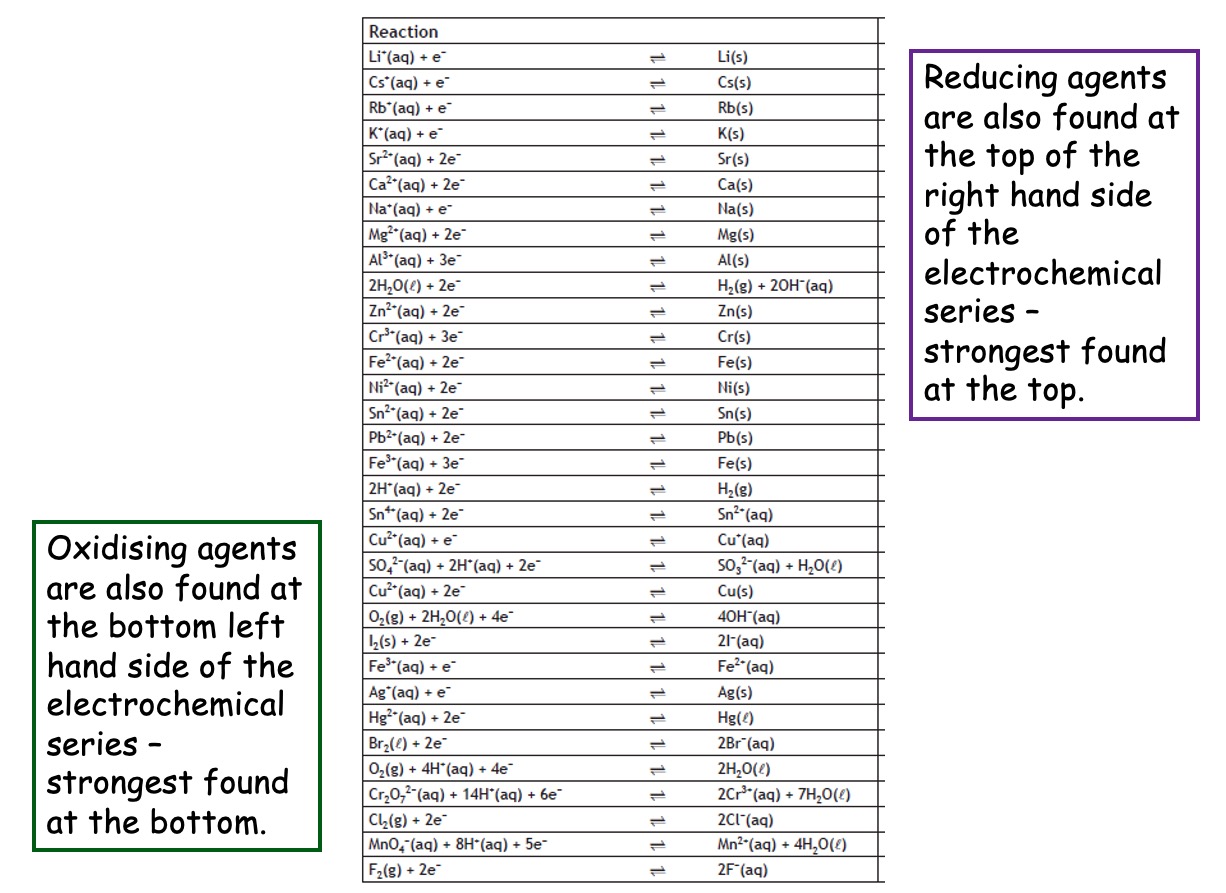
Oxidising Agents
Oxidising agents are substances that cause another species to oxidise. Oxidising agents are reduced. They specialise in electron removal, this means they accept electrons from other substances.
Elements with high electronegativies (non-metals) tend to form ions by gaining electrons (reducting) so can act as oxidising agents.
Reducing Agents
Reducing agents are substances that they cause another species to reduce. Reducing agents are oxidised. They specialise in electron donation, this means they donate electrons to other substances.
Elements with low electronegativities (metals) tend to form ions by losing electrons (oxidising) so can act as reducing agents.
Oxidising agents are widely used because of the effectiveness with which they can kill fungi and bacteria, and can inactivate viruses. The oxidation process is also an effective means of breaking down coloured compounds, making oxidising agents ideal for use as 'bleach' for clothes and hair.
In the periodic table, the strongest reducing agents are in group 1, and the strongest oxidising agents are in group 7.
Displacement reactions are examples of redox reactions.
E.g. Zinc metal displaces silver from silver (I) nitrate solution. This is because zinc is higher than silver in the electrochemical series.
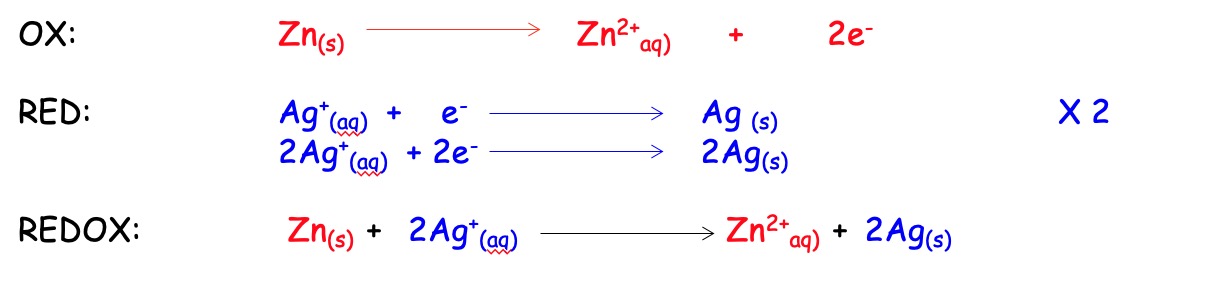
The zinc metal is the reducing agent. It donates electrons and it is oxidised.
The silver ion is the oxidising agent. It accepts electrons and it is reduced.
Oxidation cannot happened without reduction and vice versa. Combining the two equations produces a REDOX equation.
The number of electrons lost in the oxidation must balance the number of electrons gained in the reduction.
Redox equations never contain electrons.
NB -The nitrate ions do not appear in the redox equation. This is because they are spectator ions and are not directly involved in the electron transfer.
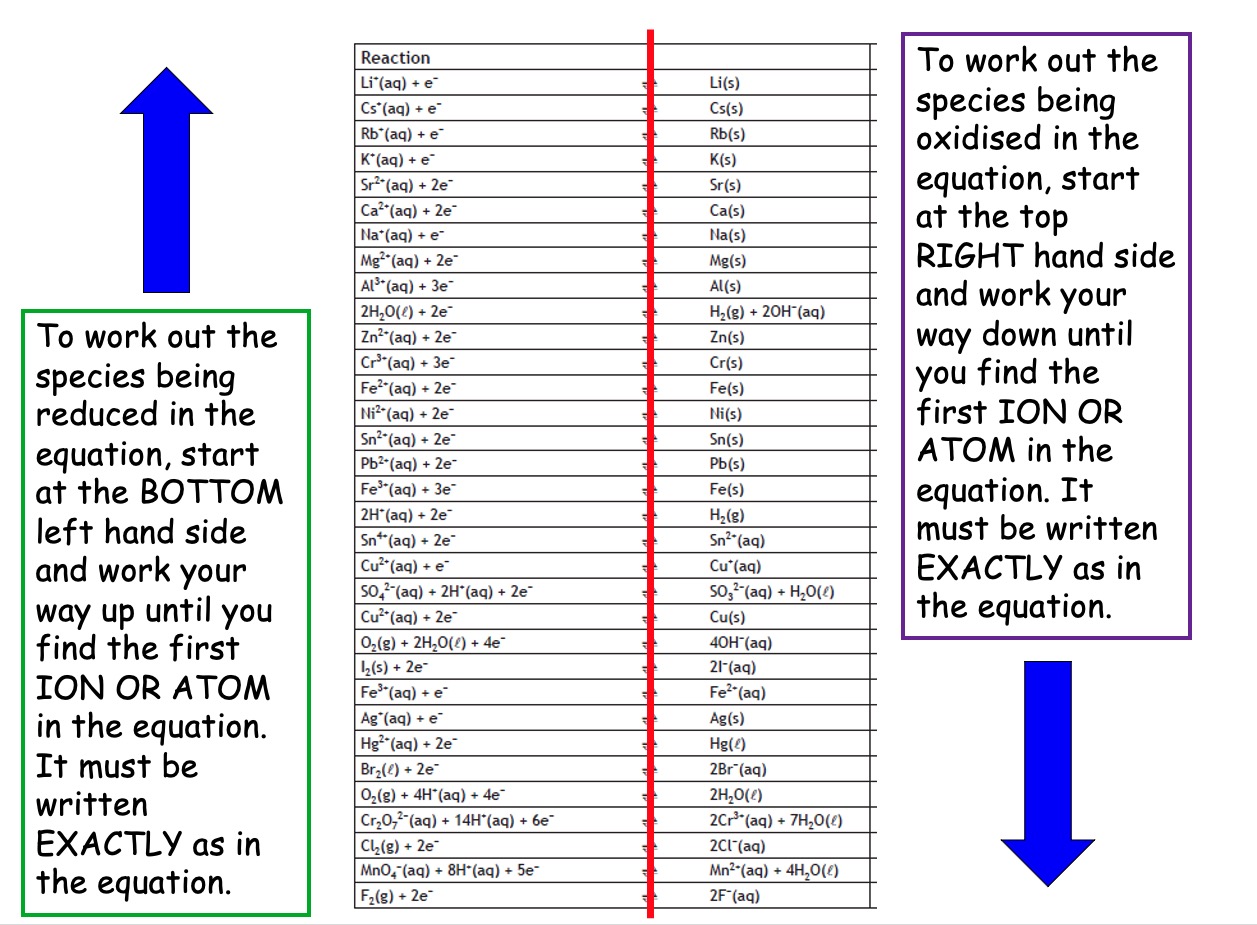
Oxidation and reduction equations can be represented by ion-electron equations. When molecules or group ions are involved, the ion-electron equation is a little more complicated.
Some are given in the data book but some you will have to write yourselves using the rules.
Potassium permanganate (KMnO4) oxidises Fe2+ ions to Fe3+ ions. During this reaction the MnO4- ions are reduced to Mn2+ ions.
a) Complete the reduction half equation:
b) Write the redox equation for the reaction.
Complete the ion-electron equation for the reduction reaction:
NO3- → NO
Write the redox equation for the reaction between bromine and sodium sulphite. In this reaction sodium (Na+) is a spectator ion.
The correct redox equation for the reaction of bromine with a solution containing iodide ions is:
In an acidic solution it is possible to convert vanadium ions to vanadate ions i.e.
V3+ (aq) → VO43- (aq)
To balance this ion electron equation the number of electrons needed is
A titration is a technique which can be used to determine the concentration of a substance, based on known concentrations and volumes of other reactants.
For many reactants an indicator is required to identify the end-point of the reaction, however for some redox titrations the end-point can be recognised from a colour change in one of the reactants (self - indicating) eg. potassium permanganate solution, the purple permanganate ions are reduced to colourless manganese ions.
A standard 250cm3 solution of vitamin C was prepared. 25cm3 of this solution was titrated against 0.034mol l-1 iodine solution using starch as an indicator. The average titre was 16.45cm3. Calculate the mass of vitamin C in the original tablet. The formula mass of vitamin C is 176g.
C6H8O6 + I2 → 2I- + C6H6O6 + 2H+
A standard 250cm3 solution of vitamin C was prepared. 25cm3 of this solution was titrated against 0.031mol l-1 iodine solution using starch as an indicator. The average titre was 17.6cm3. Calculate the mass of vitamin C in the original tablet.
C6H8O6 + I2 → 2I- + C6H6O6 + 2H+
25cm3 of acidified potassium permanganate solution reacts with 23.7cm3 of a 0.01mol l-1 solution of iron(II) sulfate. Calculate the concentration of potassium permanganate solution.
MnO4- + 8H+ + 5Fe2+ → Mn2+ + 4H2O + 5Fe3+
The following reactions take place when nitric acid is added to zinc.
NO3- (aq) + 4H+ (aq) +3e- → NO (g) + 2H2O (l)
Zn (s) → Zn2+ (aq) + 2e-
How many moles of NO3- (aq) are reduced by one mole of zinc?
During a redox process in acid solution, iodate ions are converted into iodine.
To balance the equation, what is the value of x?
2IO3- (aq) + 12H+ (aq) +xe- → I2(aq) + 6H2O (l)
Redox Equations Part 1
Redox Equations Part 2