

There are four main types:
In order of strongest to weakest:
There are three types of covalent boding in compounds
Covalent Networks

These covalent network compounds have the same properties as covalent network elements. Both SiC and SiO2 have very high melting points as melting requires breaking strong covalent bonds. Silicon carbide (structurally similar to diamond) has many uses due to it's strength, durability and low cost. Silicon carbide is often referred to as 'carborundum'
Covalent Molecules
A covalent bond is formed when atoms of elements share electrons.
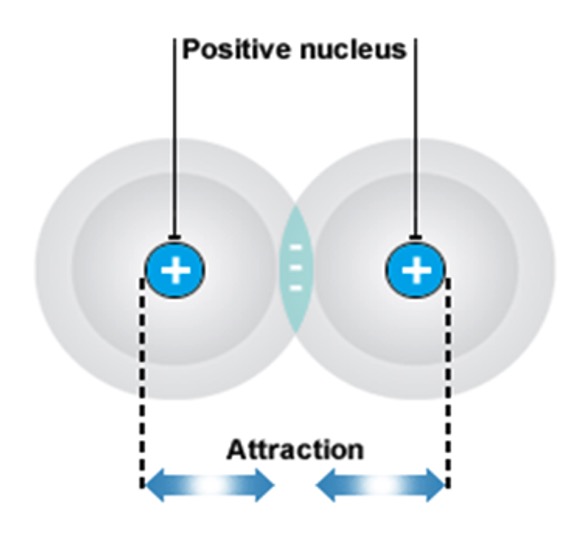
There are two types of covalent bond:
Covalent bonds are strong (100 to 500 kJ mol-1).
Non-Polar Covalent
A pure covalent bond is formed when the atoms involved in the bond have an equal share of the bonding electrons ie. equal electronegativity.
All diatomic elements contain pure covalent bonds as both atoms have the same electronegativity value.
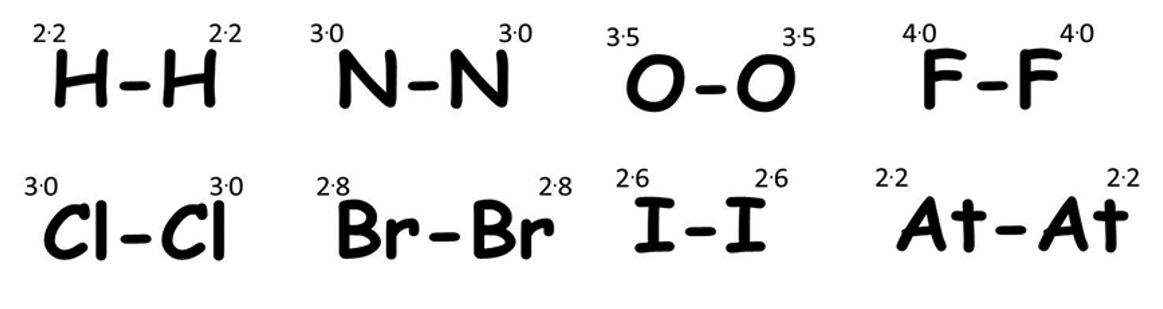
Polar Covalent
When atoms with different electronegativity values (approximately between 0.5 and 1.6), join together, a polar covalent bond is formed.
In a polar covalent bond the electrons are not shared equally, one atom in the bond has a greater attraction than the other for the bonded electrons.
Example: HBr
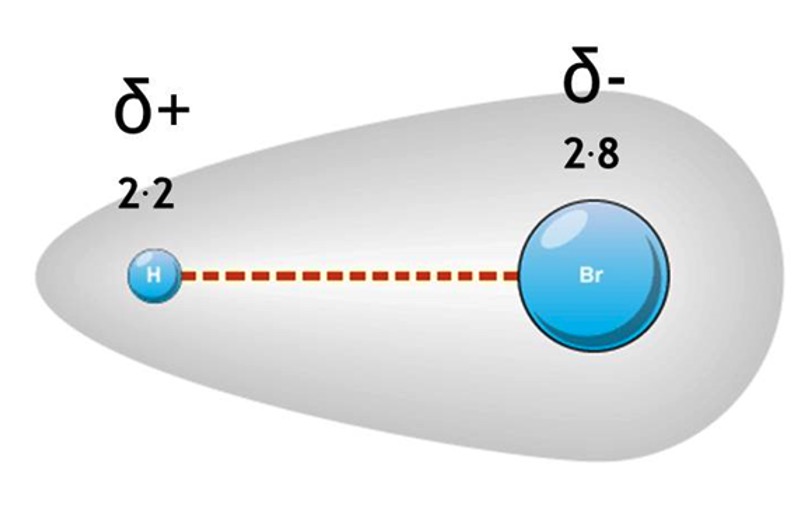 Hydrogen electronegativity - 2.2
Hydrogen electronegativity - 2.2
Bromine electronegativity - 2.8
Br end becomes slightly negatively charged δ-
H end becomes slightly positively charged δ+
A polar covalent bond is a bond in which the atoms have different electronegativity values.
This means that one atom has a greater attraction for the bonded electrons than the other.
δ- means slightly negative (most electronegative atom)
δ+ means slightly positive (least electronegative atom)
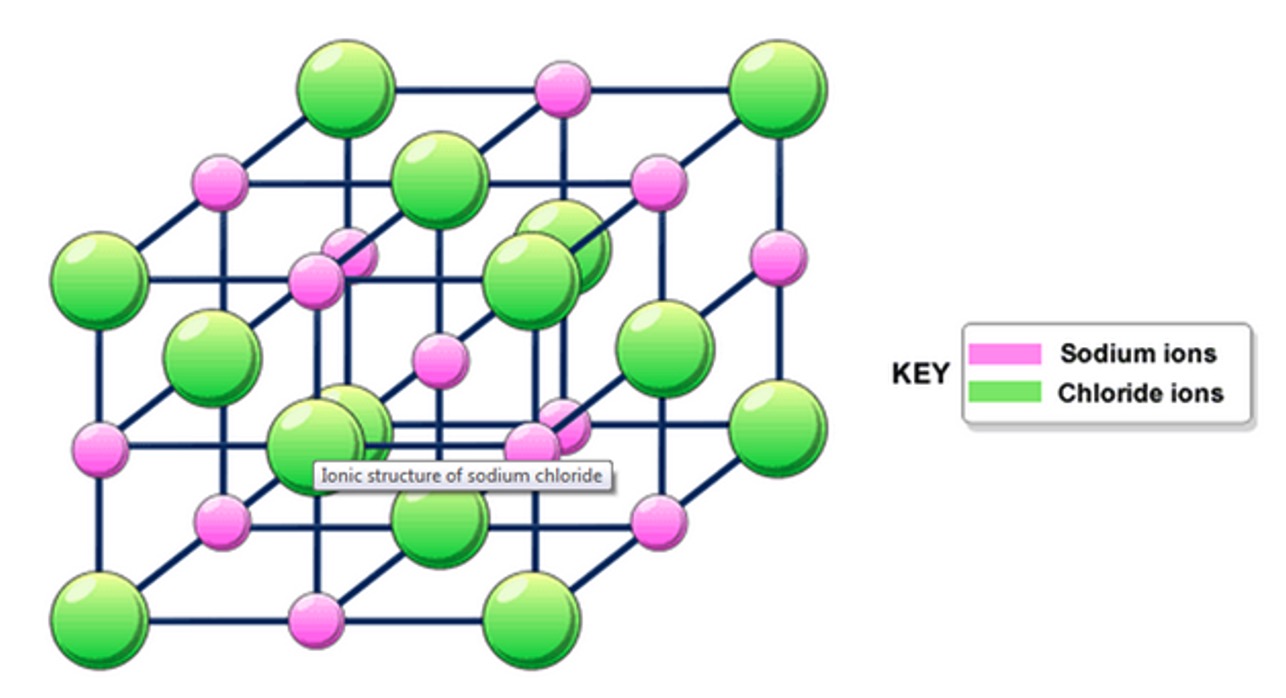
Ionic bonding is the electrostatic attraction between the positive ions of one element and the negative ions of another element.
The greater the electronegativity difference is between the two elements, the more likely they are to form an ionic bond (difference in ΔEN is greater than 2.0).
The element with the larger electronegativity will gain electrons to form a negatively charged ion, whilst the less electronegative element will lose electrons to form a positively charged ion.
Elements far apart in the Periodic Table are most likely to form ionic compounds.
This means that ionic compounds typically arise from metals combining with non-metals.
Ionic bonds are strong (100 to 450 kJ mol-1) and ionic compounds exist as crystal lattice structures in which each ion is attracted to a number of oppositely charged ions.
A bonding continuum can be used to help us understand the differences in bonding as it is not as simple a labelling compounds as ionic, pure covalent or polar covalent.
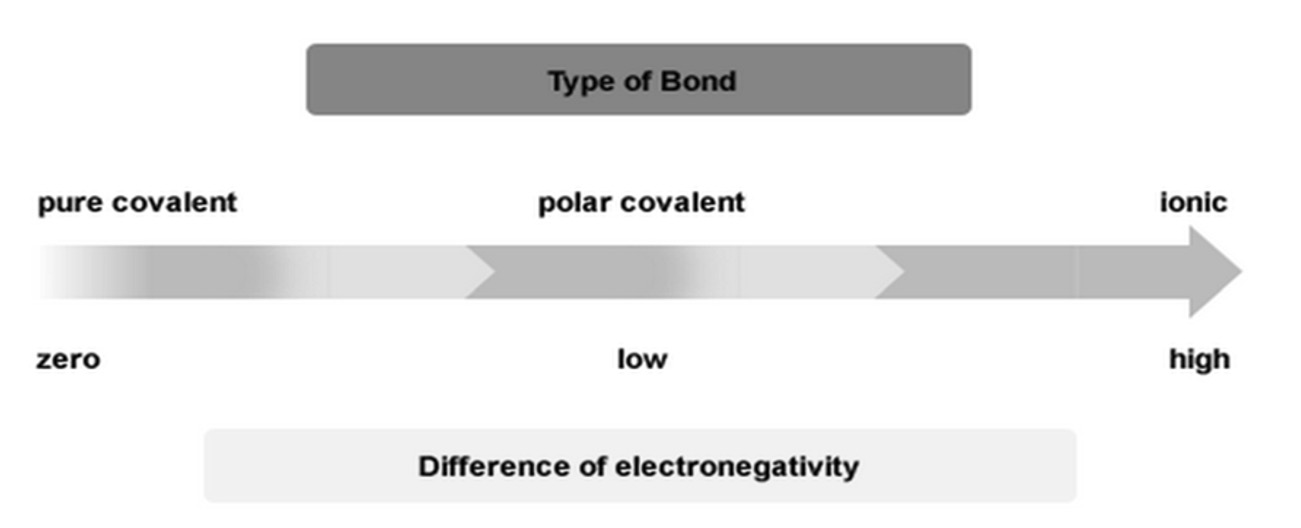
In general:
Intermolecular bonds are bonds which occur between molecules. Intermolecular bonds are called van der Waals' forces.
There are three types of van der Waals' forces:
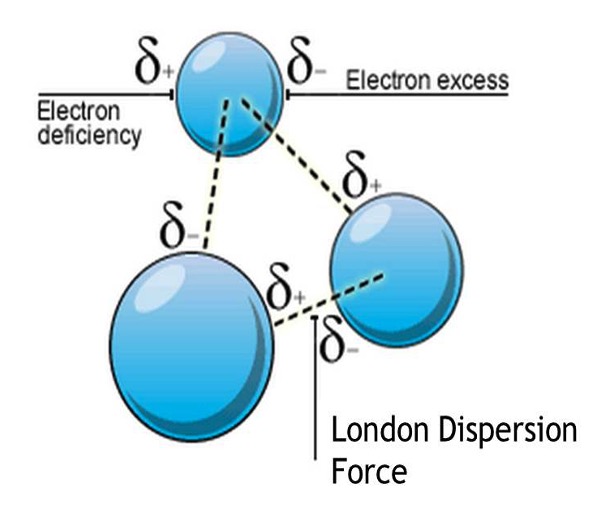 London dispersion forces are the weakest type of van der Waals' force. London dispersion forces occur between all atoms and molecules.
London dispersion forces are the weakest type of van der Waals' force. London dispersion forces occur between all atoms and molecules.
London dispersion forces are formed as a result of electrostatic attraction between temporary dipoles and induced dipoles caused by movement of electrons.
There is an uneven distribution of the constantly moving electrons around the nuclei of atoms.
This movement causes the formation of temporary dipoles on the atoms.
The temporary dipole formed means that atoms then attract each other.
These temporary dipoles constantly change, but always exist.
The larger an atom or molecule is, the greater the number of London dispersion forces that can occur.
The strength of the London dispersion force is related to the number of electrons within an atom or molecule.
Permanent Dipole- permanent dipole interactions occur between polar molecules. Dipole-dipole interactions are stronger than London dispersion forces.
A dipole-dipole interaction is the electrostatic attraction between the oppositely charged areas of two or more polar molecules. Think of the electrons pulled to one side of the molecule where one side is slightly positive and the other slightly negative.
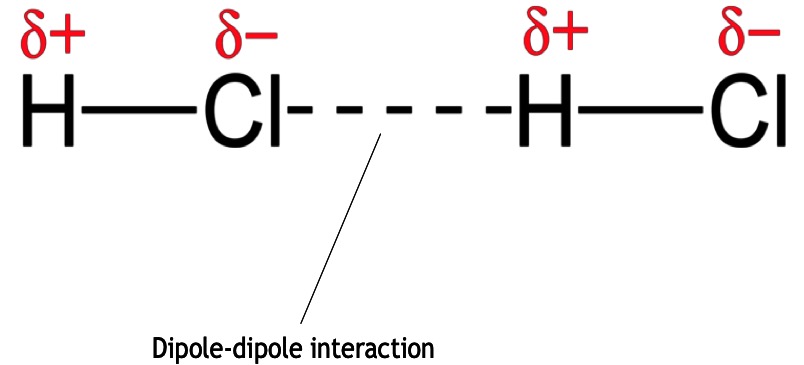
Permanent dipole to dipole interaction is caused via the constant attraction between the δ+ atoms and δ- atoms of neighbouring molecules. A molecule is described as polar if it has a permanent dipole.
Due to dipole - dipole interactions being stronger than London dispersion forces, polar molecules have higher melting and boiling points than non-polar molecules.
Polar or Non-Polar?
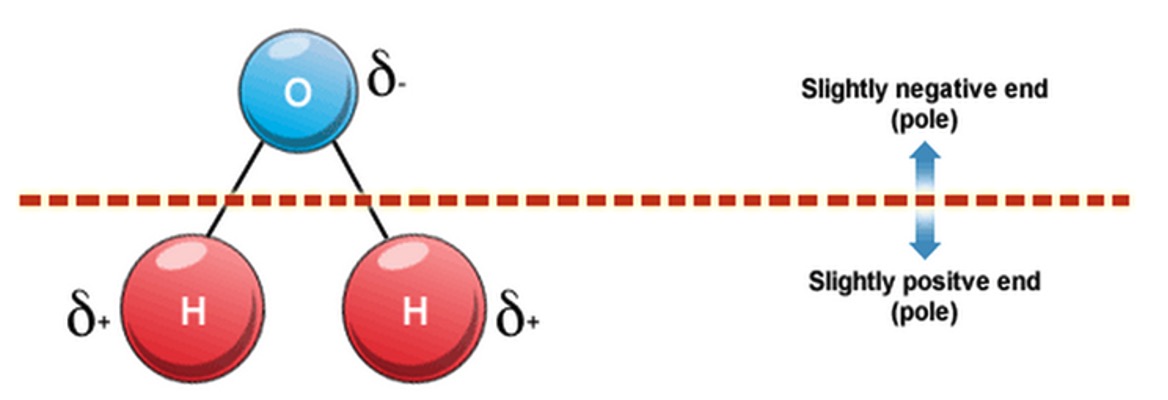
Some molecules contain polar covalent bonds and are described as polar molecules as they are not arranged symmetrically and negative poles and positive poles exist in the molecule.
e.g. water
Other molecules are non-polar even although they contain polar covalent bonds, as they are symmetrical so there are no positive or negative poles in the molecule.
e.g. Tetrachloromethane CCl4
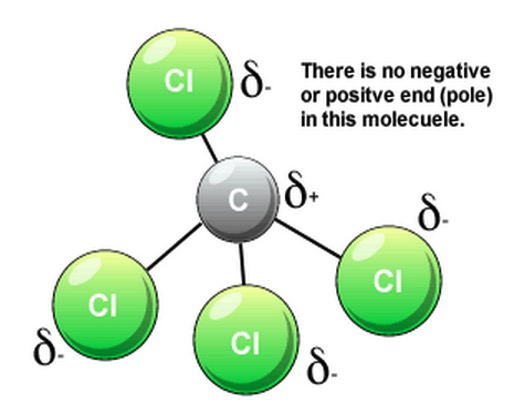 In this molecule the symmetry causes the polarity to cancel out over the molecule as a whole.
In this molecule the symmetry causes the polarity to cancel out over the molecule as a whole.
RMEMBER:
Symmetrical: Non-polar molecule
Asymmetric: Polar molecule
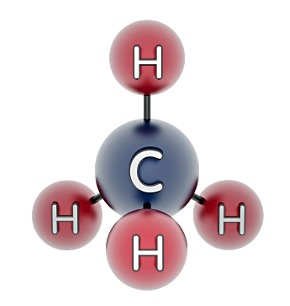
2. Methane
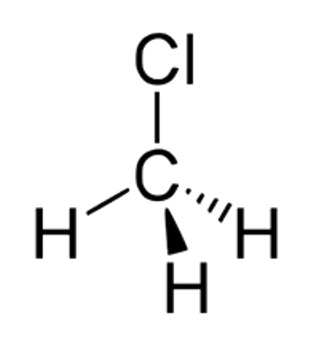
3. Chloromethane
4. Carbon dioxide
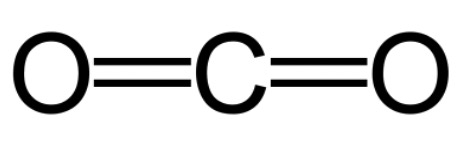
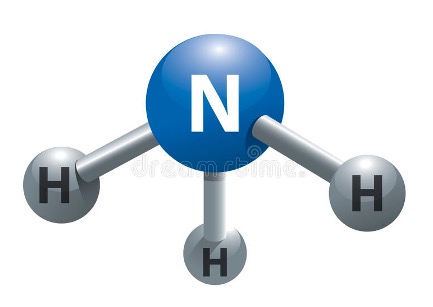
5. Ammonia
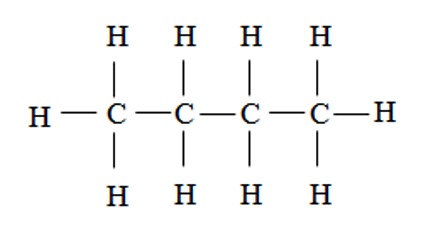
6. Butane
7. Identify the three compounds which contain polar molecules.
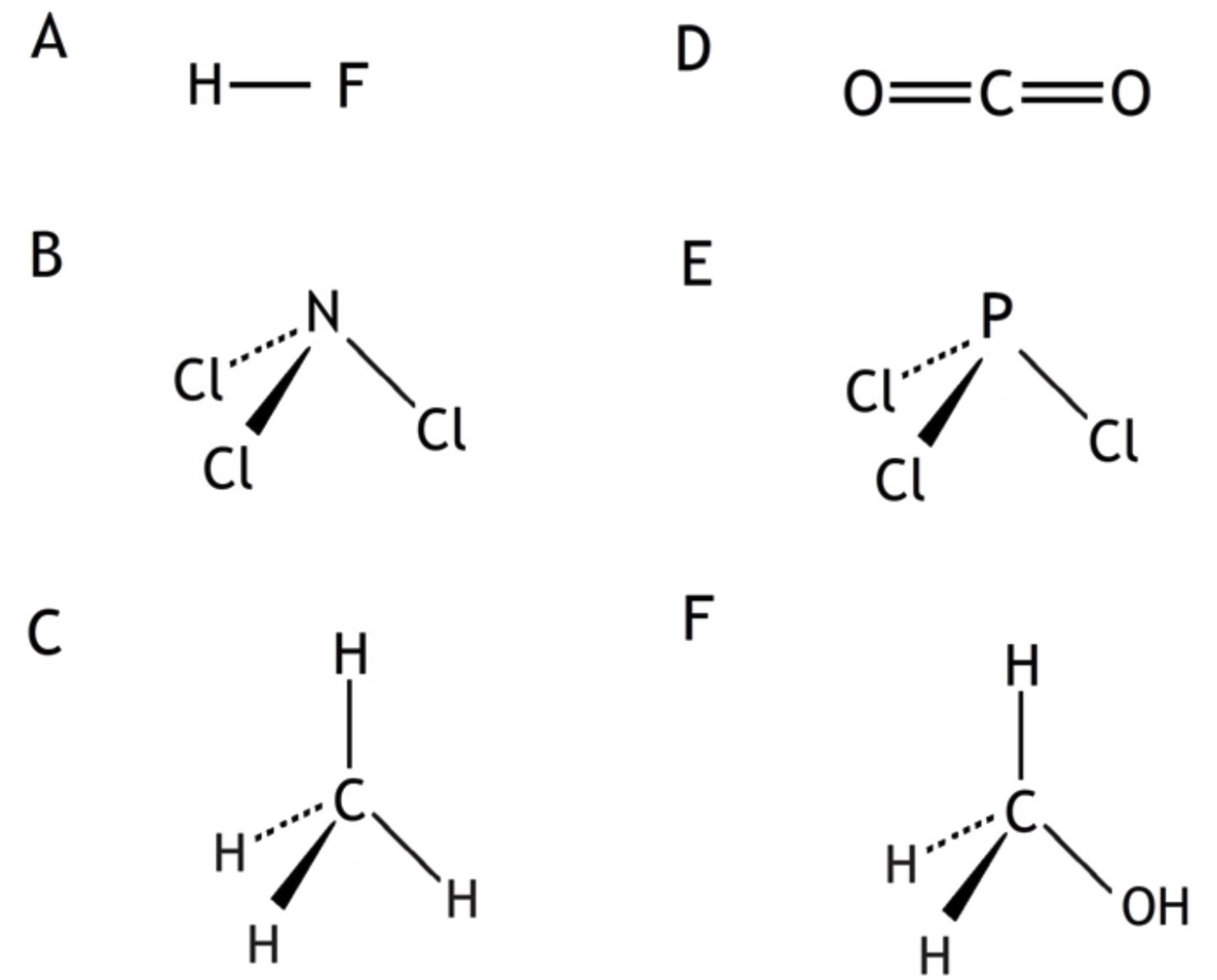
8. Atoms of nitrogen and element X form a bond in which the electrons are shared equally. Element X could be
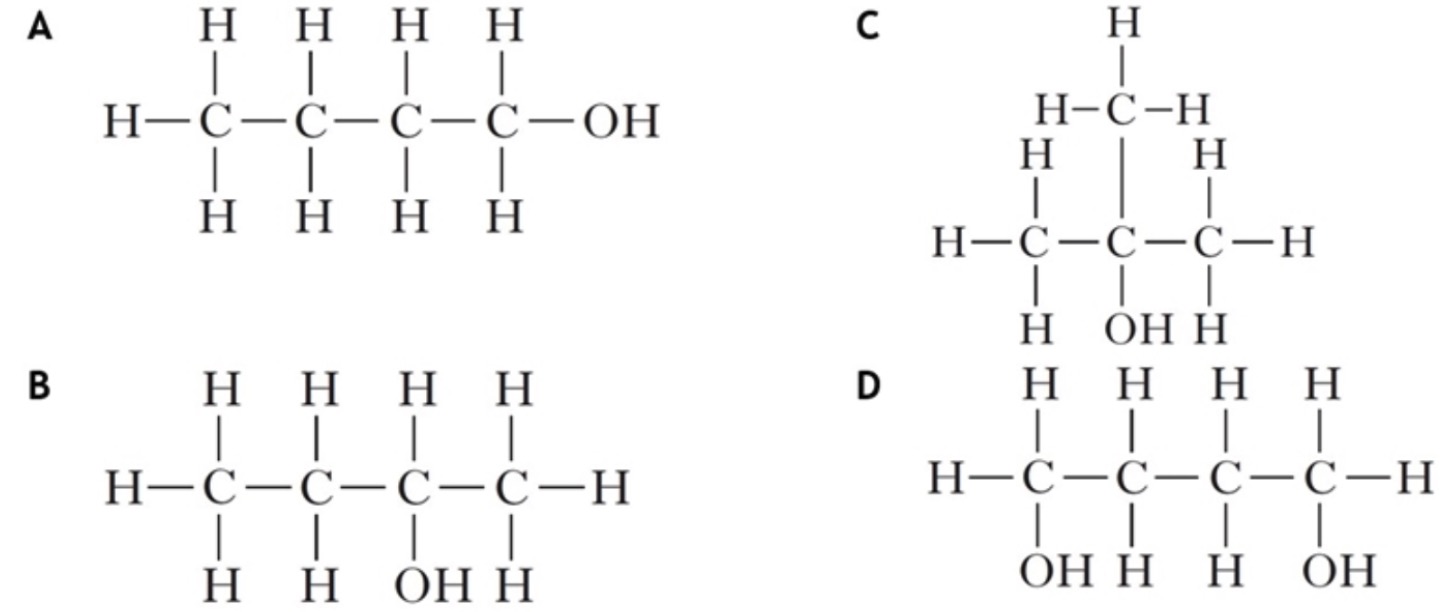
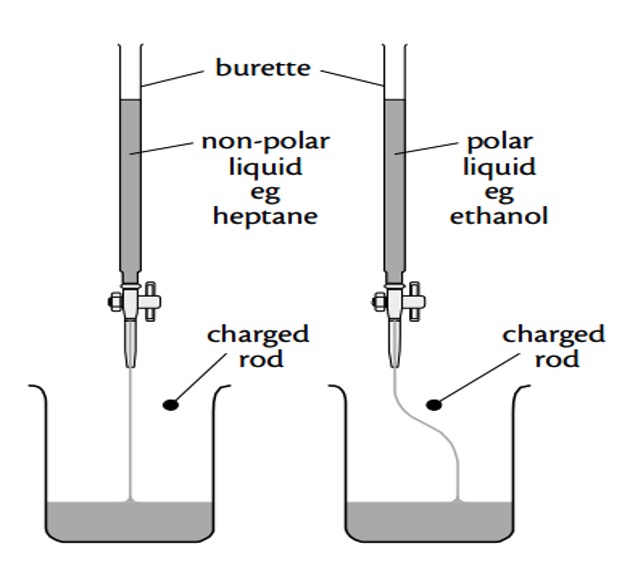 Polarity Experiment
Polarity ExperimentYou can experimentally determine if a molecule is polar or non-polar by placing a charged rod next to a flow of the substance in question.
If it is attracted towards the charged rod, it is a polar molecule.
If it is not attracted to the rod it is a non-polar molecule.
Hydrogen bonding is a special type of dipole-dipole interaction involving; H-N, H-O or H-F bonds.
These bonds are very polar, as shown in by the electronegativity values in the table below.
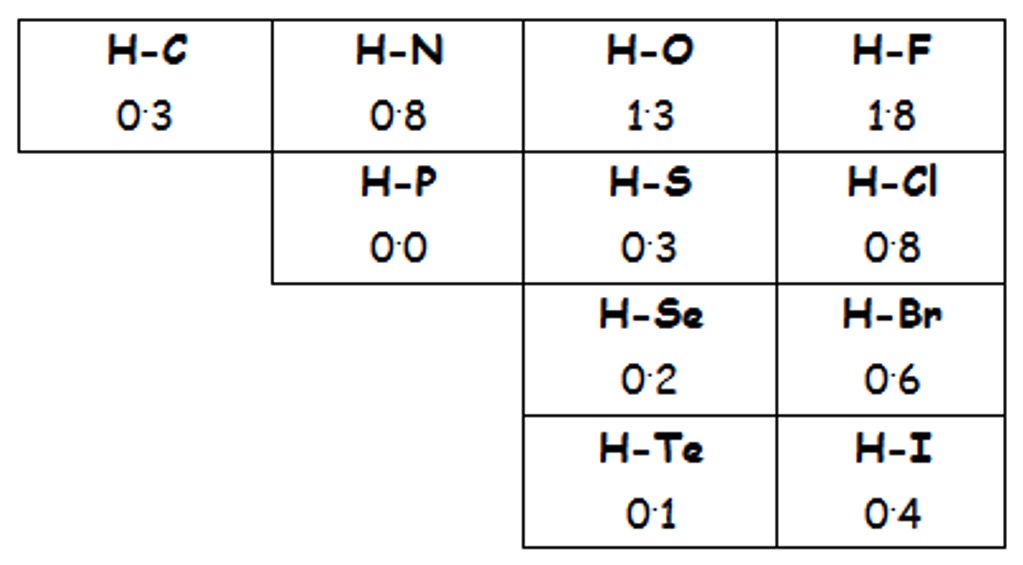
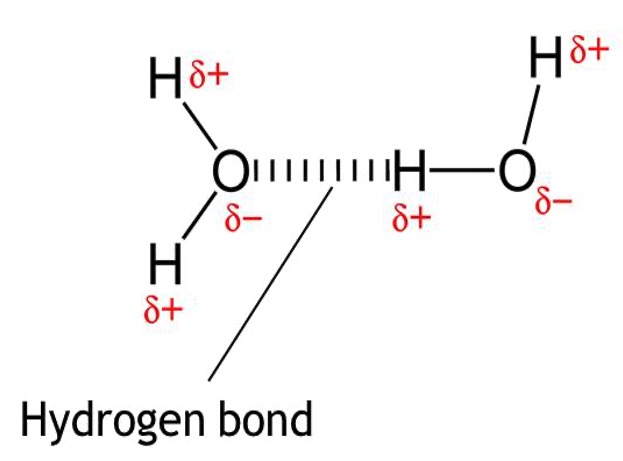 When a highly electronegative element such as nitrogen, oxygen or fluorine is bonded to hydrogen, the hydrogen ends up being slightly positively charged and the electronegative element becomes slightly negatively charged.
When a highly electronegative element such as nitrogen, oxygen or fluorine is bonded to hydrogen, the hydrogen ends up being slightly positively charged and the electronegative element becomes slightly negatively charged.
The most common example of hydrogen bonding is in water.
 London Dispersion Forces
London Dispersion Forces
Occur between all atoms and molecules but more significant in non-polar molecules.
PD/PD Interactions
Occur between polar molecules with permanent dipoles.
Hydrogen Bonding
Occur between polar molecules with H of one molecule and N, O or F of another.
Molecules which contain hydrogen bonding have much higher melting and boiling points than those with dipole-dipole interactions or London dispersion forces.
The melting and boiling point of a substance gives an indication of the quantity of energy needed to overcome the van der Waals' forces between molecules.
For non-polar molecules the melting and boiling are related to the London dispersion forces between molecules.
The strength of London dispersion forces increases with increasing molecular mass.
For polar molecules, the melting and boiling points are higher than those of non-polar compounds due to more energy being required to overcome the dipole-dipole interactions of polar molecules.
Compounds which contain H-N, H-O or H-F bonds will have significantly higher melting and boiling points compared to other polar molecules and non-polar molecules due to more energy being required to overcome the strong hydrogen bond.
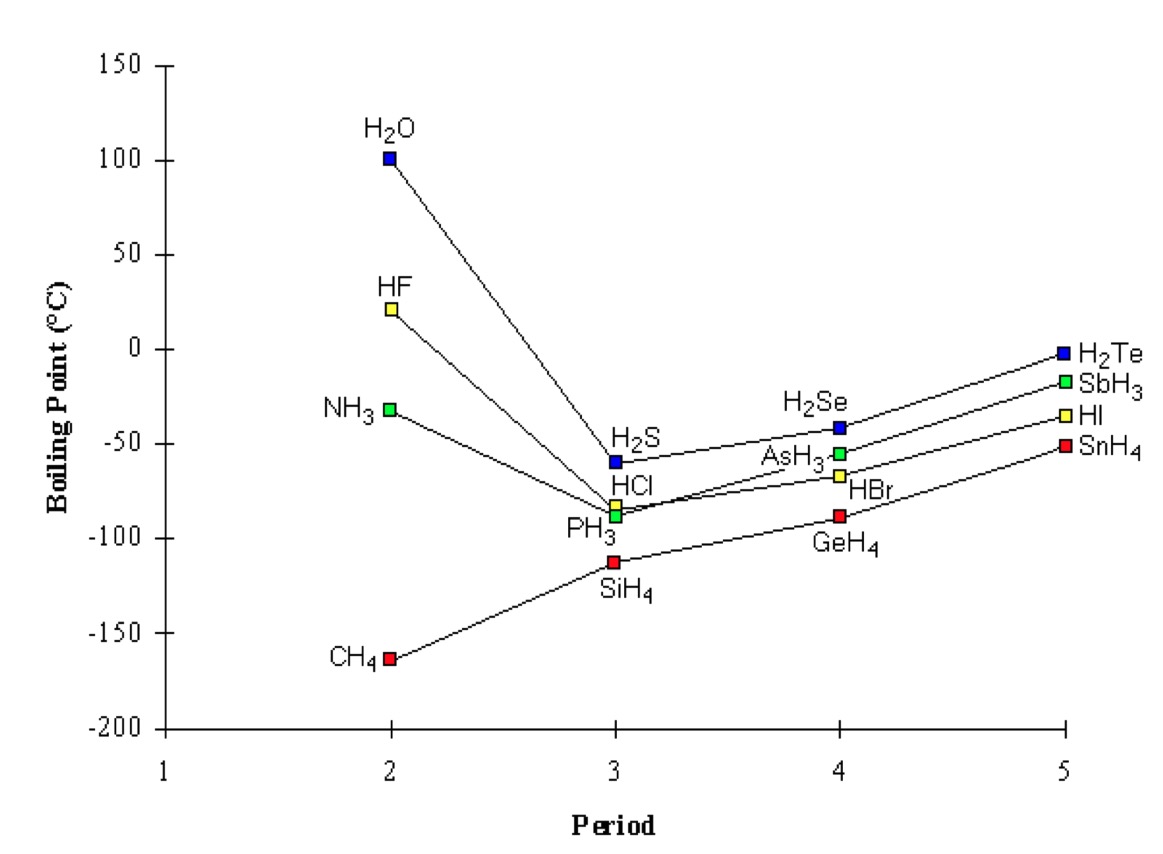
Water, hydrogen fluoride and ammonia have a boiling point higher than expected due to hydrogen bonding.
Water is unusual as it's solid form (ice) is less dense than it’s liquid form (water). This means that ice floats on water whereas most other solids sink in their own liquid forms. This phenomenon is due to the structure and bonding which takes place between the water molecules.
As water molecules cool, they contract. However, at 4oC they begin to expand. This is because of hydrogen bonds between the water molecules. This decreases the density of ice (greater volume/same mass) compared to that of the liquid water.
Ice floating is vital to 'real life - i.e. fish/marine life surviving under frozen lakes.
'like substances dissolve in like substances'
This means that polar molecules will dissolve in polar solutions but not in non polar solutions and vice versa.
This is due to the attraction between δ+ and δ- atoms of the water and the polar substance.
Ionic compounds dissolve in polar solutions in a similar way due to the interaction between the ions and the δ+ and δ- atoms.
Ions surrounded by a layer of water molecules - held by electrostatic attraction - are said to be hydrated.
The stronger the van der Waals' forces between a liquid are, the more viscous it will be.
Liquids containing hydrogen bonding will be more viscous than molecules containing dipole-dipole interactions or London dispersion forces.
The most viscous liquid is propane-1,2,3-triol.
Viscosity is not only related to molecular mass but also to Hydrogen bonding. The -OH groups allow hydrogen bonding between the molecules and this increases the viscosity.
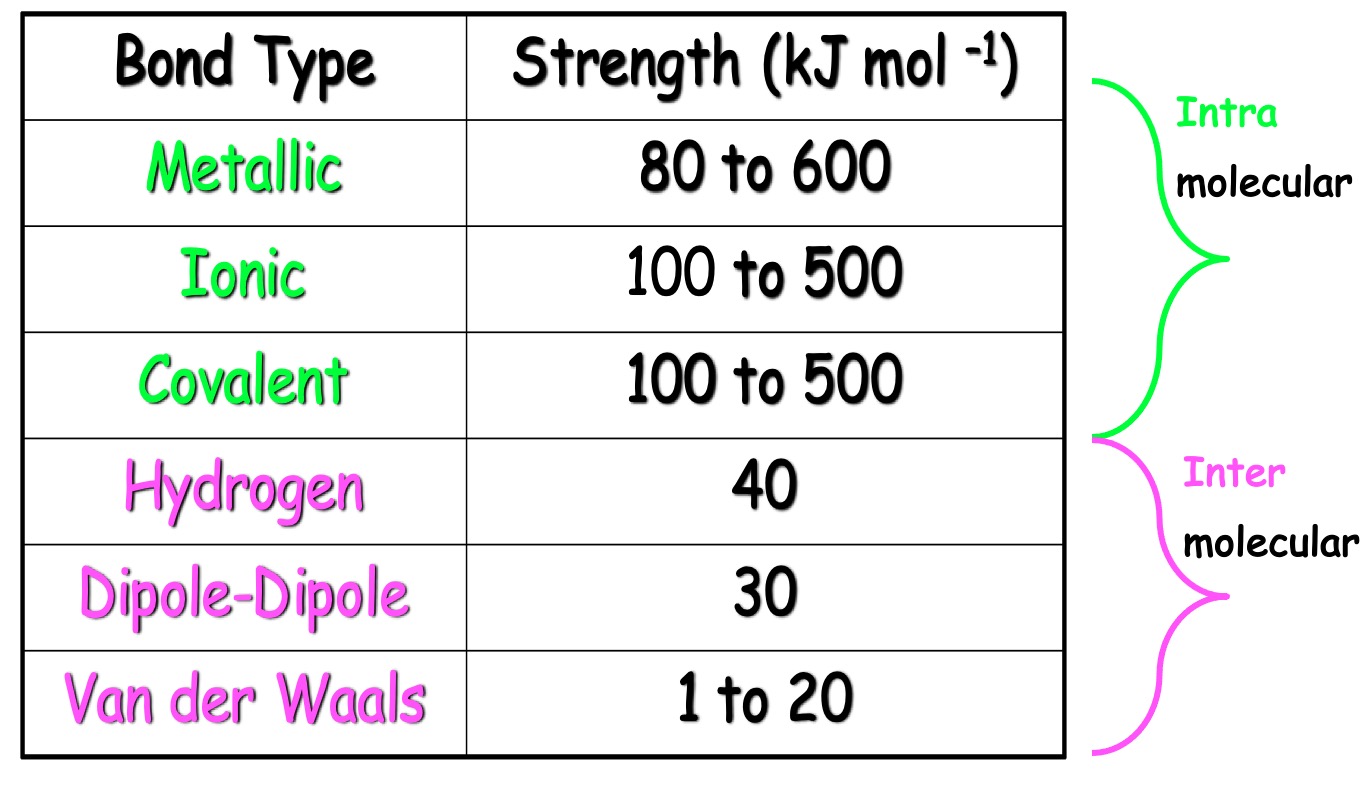
10. The structures for molecules of four liquids are shown below. Identify which liquid will be the most viscous.
11. The polarity of molecules can be investigated using a charged rod. The charged rod will attract a stream of polar liquid flowing from a burette.
Which of the following liquids would not be attracted?
Part 1
Part 2
Part 3