There are 3 types:
a) Molecular
b) Network
Intra - means within.
In other words an Intramolecular bond means bonds within the molecule. Examples of this are metallic, ionic and covalent bonds.
Inter - means in between.
In other words an Intermolecular bonds means bonds in between the molecules. We will look at examples of these types of bonds later in Unit 1.
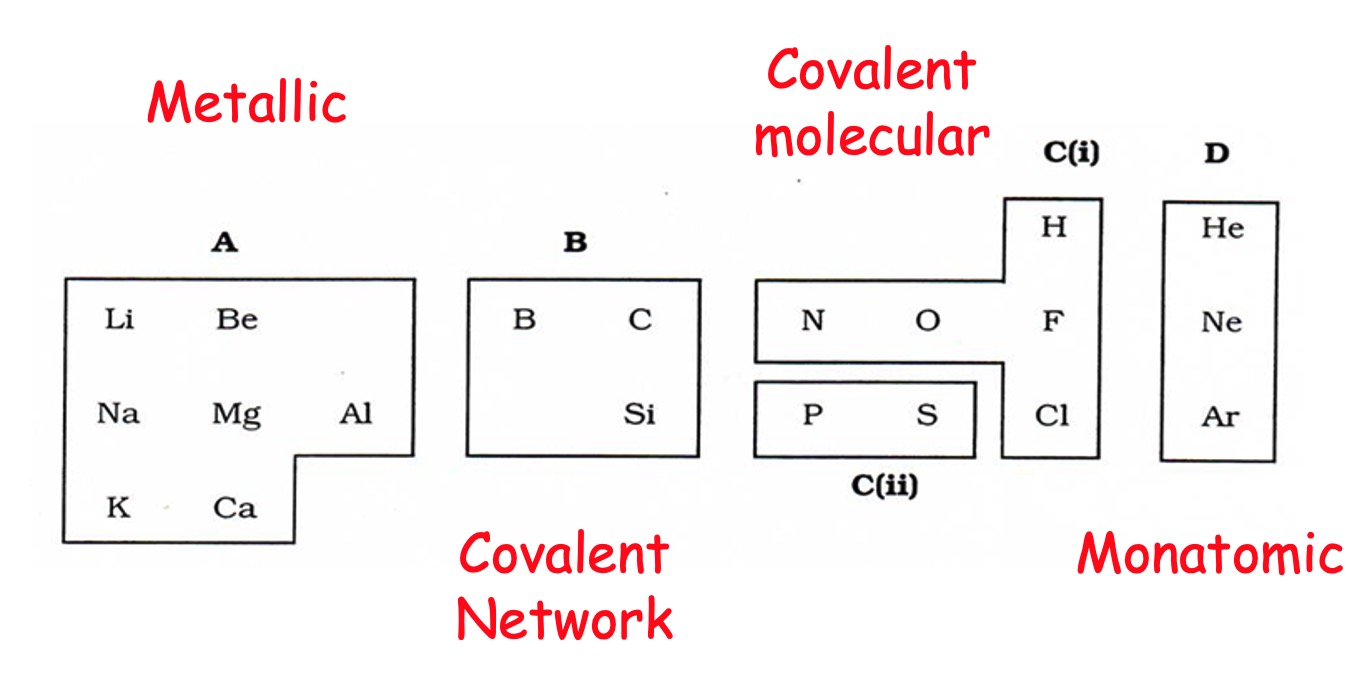
Metallic bonding - unsurprisingly - only appears in metal elements.
Metallic bonding occurs between (positively charged) metal ions and delocalised outer shell electrons.
'delocalised' means the electrons are common to all of the ions (i.e. they move from one to another).
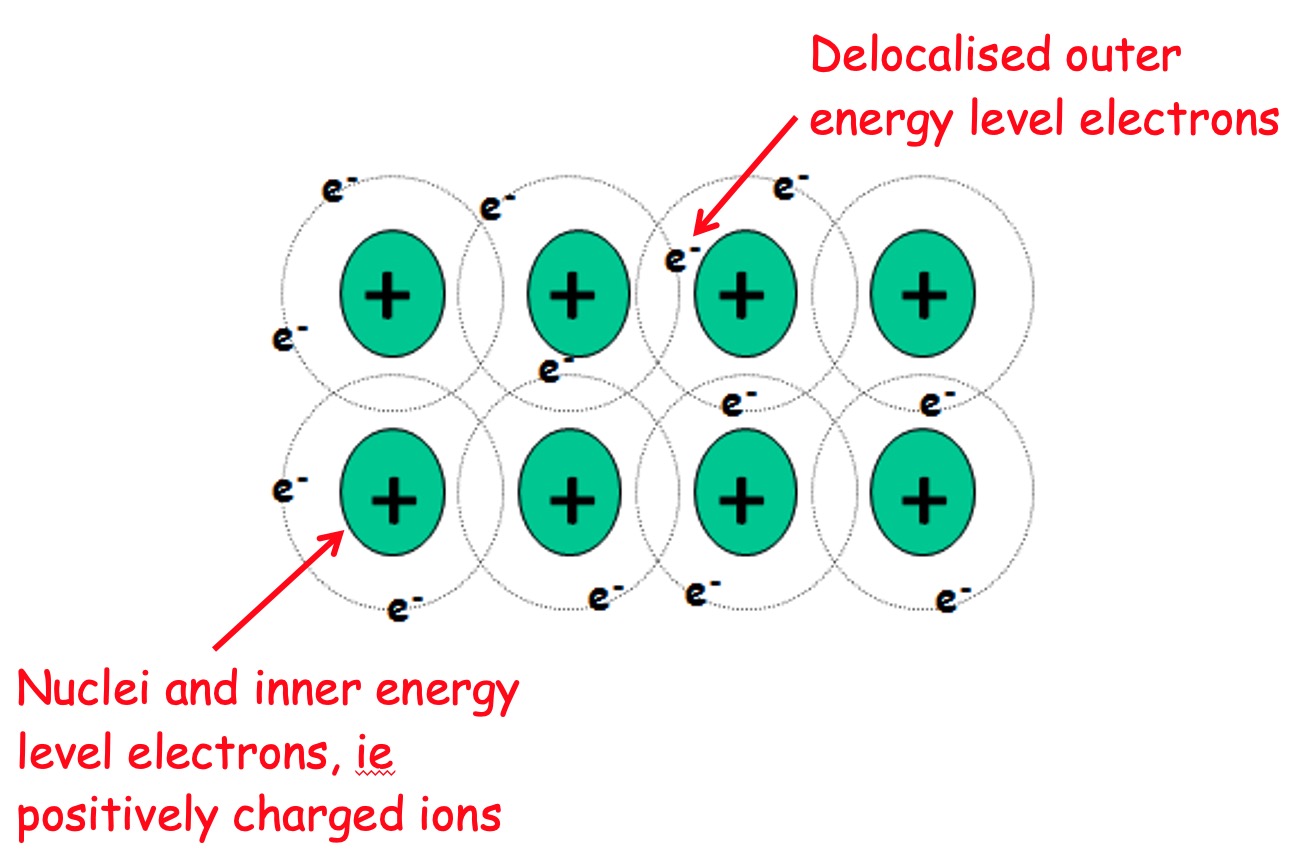
Atoms in a metal contribute the electrons in their outermost energy levels to a common 'pool' of free or delocalised electrons.
The structure of metals can be regarded as a regular array of positive charged ions in a sea of delocalised electrons.
Each positively charged ion is attracted to the pool of negative electrons and vice versa.
These electrostatic attractions, which constitute the metallic bond, hold the entire metal crystal together as a single unit.
Metallic bonds are generally strong.
The strength of the bond will largely depend on the number of electrons that each atom contributes to the delocalised pool and the charge on the ions in the array.
The greater the number of delocalised electrons and consequently the charge on the ions, the stronger the bond will be.
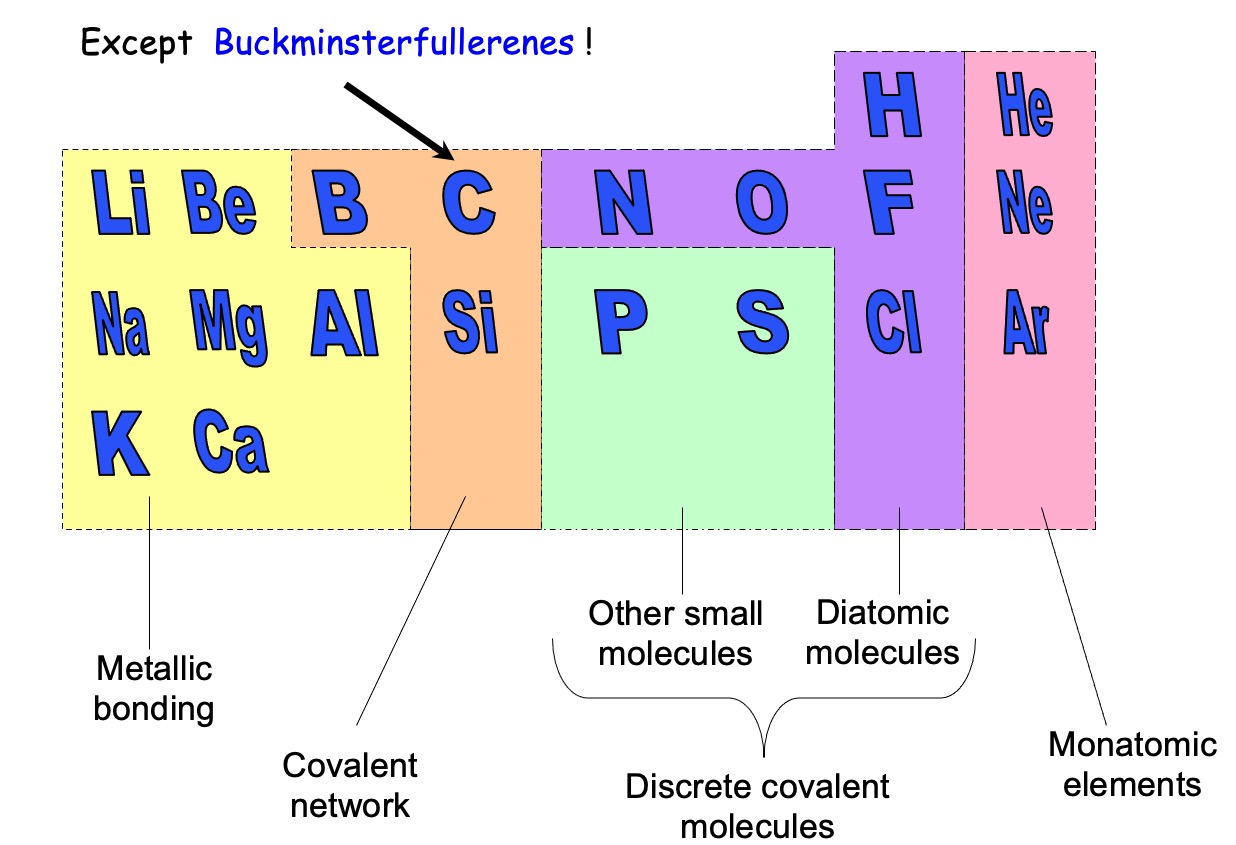
Covalent bonding occurs between two non metal atoms.
Covalent bonds are held together through the attraction between the positively charged nucleus of one atom and the negatively charged outer electrons of the other atom.
Outer electrons are shared in covalent bonding.
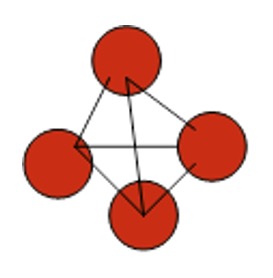 Phosphorus - forms P4 tetrahedral molecule. It has a low melting point and is solid at room temperature
Phosphorus - forms P4 tetrahedral molecule. It has a low melting point and is solid at room temperature
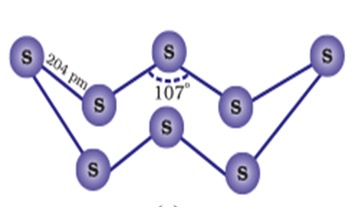 Sulphur - forms two covalent bonds using its half filled orbitals. It forms closed 8 membered rings in a zig zag form.
Sulphur - forms two covalent bonds using its half filled orbitals. It forms closed 8 membered rings in a zig zag form.
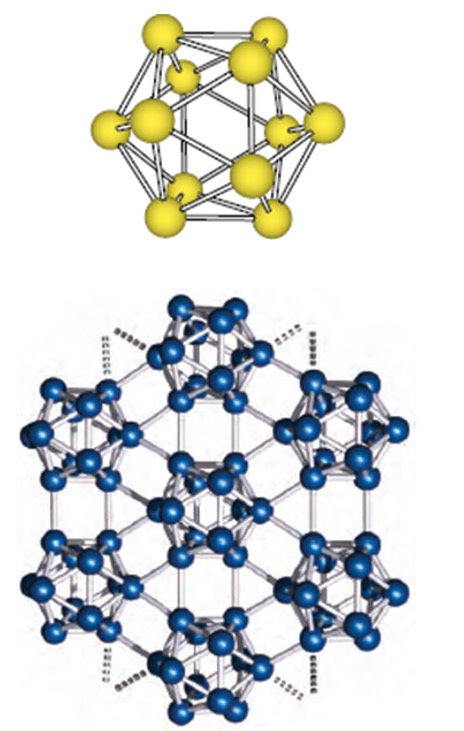
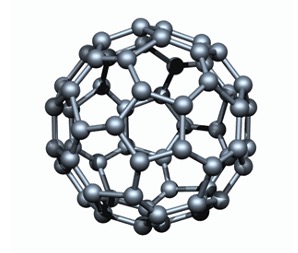 Fullerenes - contains 60 carbon atoms and is a discrete molecular solid.
Fullerenes - contains 60 carbon atoms and is a discrete molecular solid.
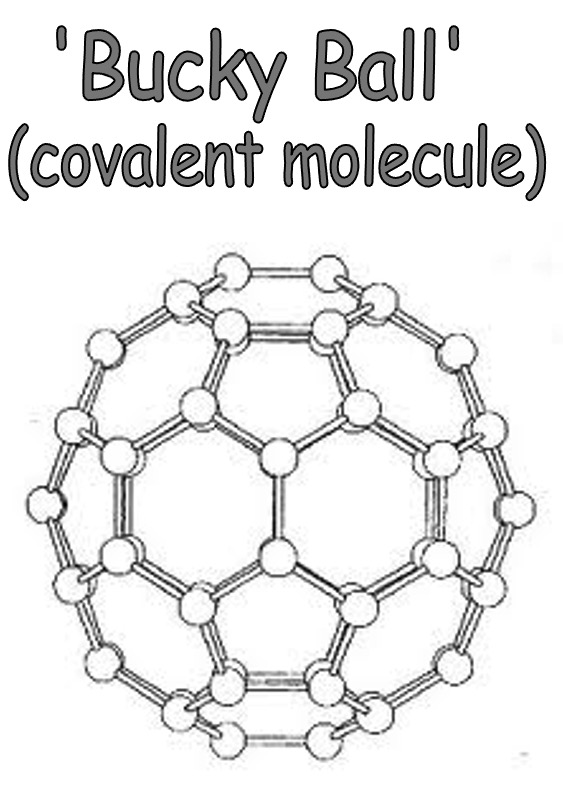 The fullerenes, despite being large molecules, are discrete covalent molecules.
The fullerenes, despite being large molecules, are discrete covalent molecules.
The smallest of fullerenes is a molecule known as Buckminsterfullerene (C60).
This is a spherical molecule containing 5 and 6 membered carbon rings.
The properties are still being researched so the full applications are still unknown.
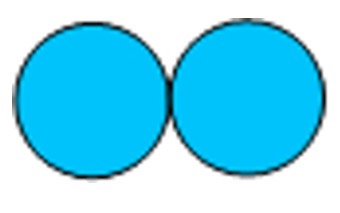 Diatomic- hydrogen, nitrogen, oxygen, fluorine and chlorine exist as diatomic gases at room temperature and are an example of discrete covalent molecular substances.
Diatomic- hydrogen, nitrogen, oxygen, fluorine and chlorine exist as diatomic gases at room temperature and are an example of discrete covalent molecular substances.
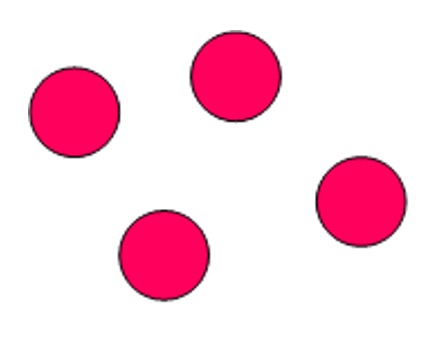 Monatomic - group 0 elements are called the 'noble gases'. The noble gases have full outer energy levels and are highly stable. As a result of their stability they have no need to form bonds and exist as single atoms which are held together by weak intermolecular forces of attraction.
Monatomic - group 0 elements are called the 'noble gases'. The noble gases have full outer energy levels and are highly stable. As a result of their stability they have no need to form bonds and exist as single atoms which are held together by weak intermolecular forces of attraction.
A giant 3D lattice of covalently bonded atoms.
Boron (group 3)
 Each atom covalently bonds to 5 other atoms.
Each atom covalently bonds to 5 other atoms.
This means covalent bonds must be broken to melt/ boil = very high m.pt/b.pt values which is why it is used to make Pyrex glassware.
No free electrons = no conduction.
Structure is almost as hard as diamond.
Carbon (group 4)
Carbon has more than one allotrope (physical form).
Diamond
 Each atom covalently bonds to 4 other atoms (tetrahedral shape).
Each atom covalently bonds to 4 other atoms (tetrahedral shape).
This means covalent bonds must be broken to melt/ boil = very high m.pt/b.pt values.
No free electrons = no conduction.
Tunnels between atoms allow light through = transparent structure.
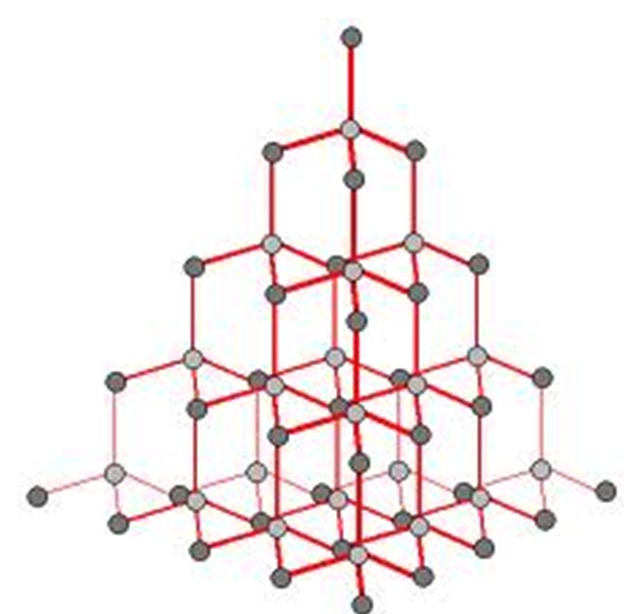
Graphite
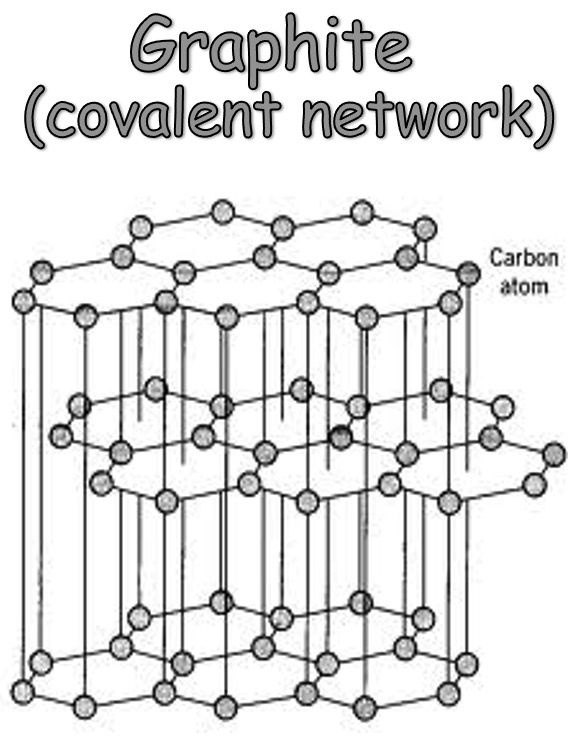 Each atom forms 3 covalent bonds and its last valence electron becomes delocalised.
Each atom forms 3 covalent bonds and its last valence electron becomes delocalised.
As the delocalised electrons are only held weakly they can flow i.e. graphite conducts electricity.
The delocalised orbitals sit between the layers - as a result there are 3 strong covalent bonds WITHIN the layers but only weak interaction BETWEEN the layers.
Due to these weak interactions, graphite is flaky as the layers can be easily separated.
Graphite layers are offset (i.e. not above each other) - light can't travel through it meaning it is not transparent.
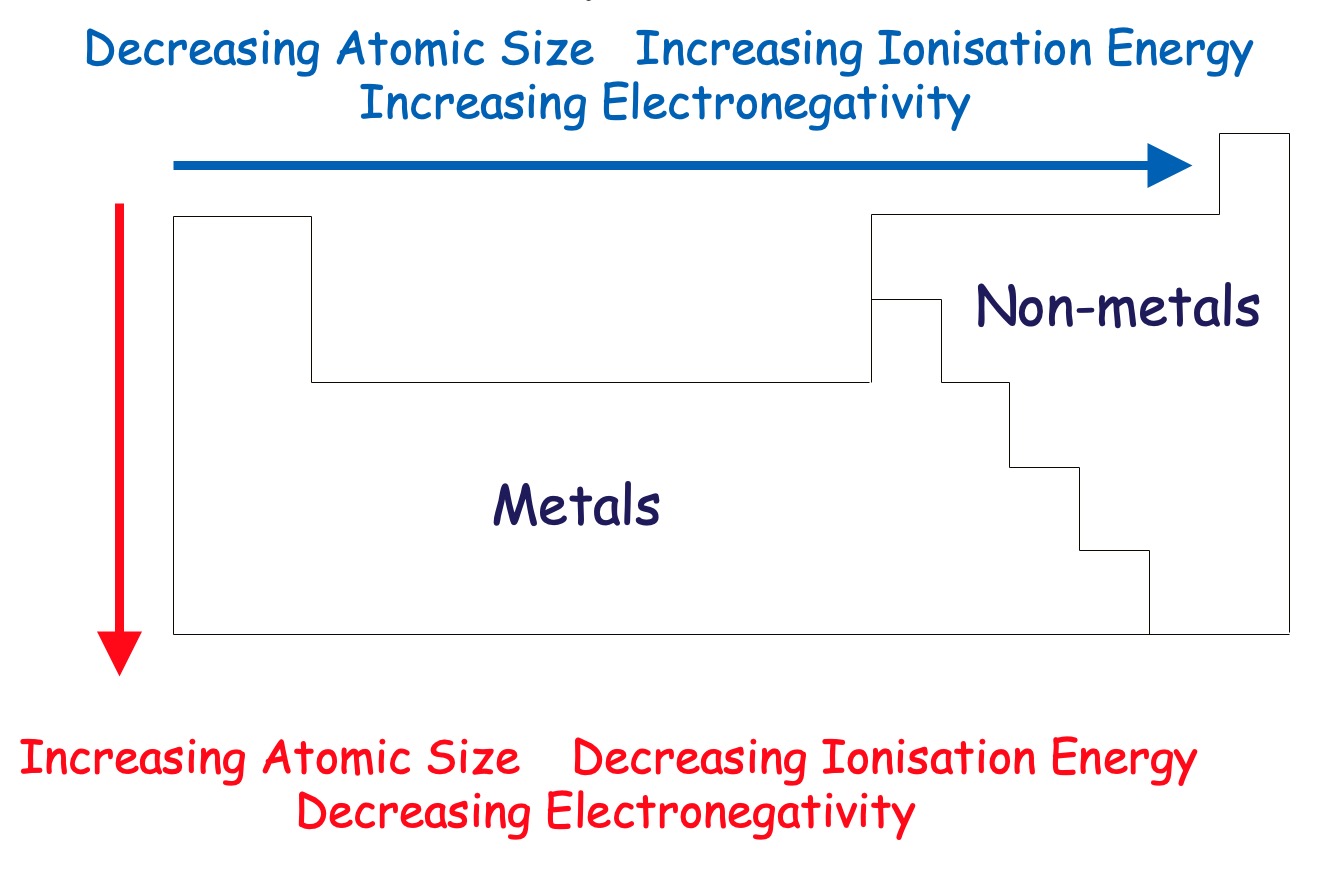
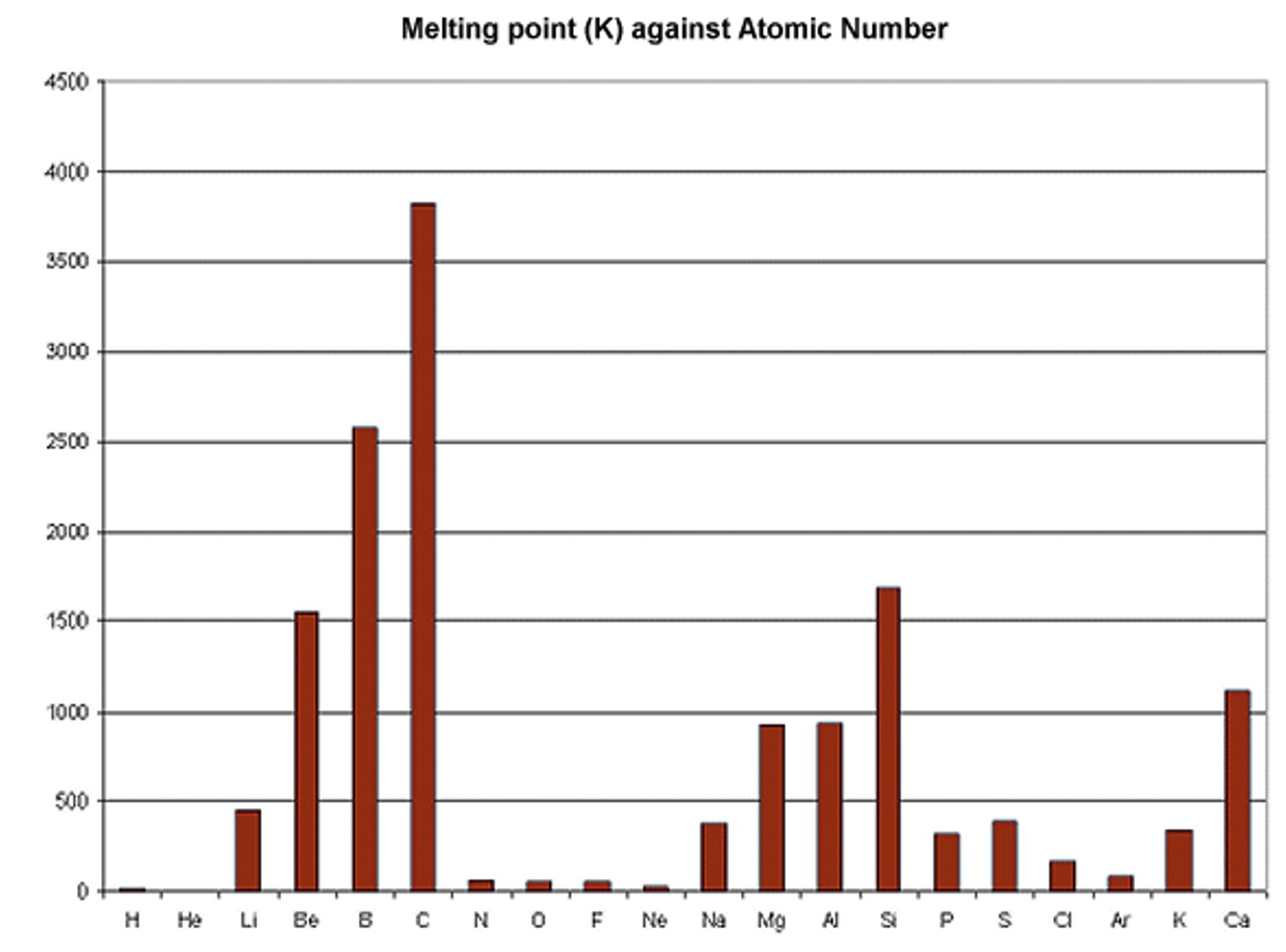
Generally, the stronger the bond between atoms, the higher the energy required to break that bond. Melting/boiling points are varied and don't generally form a trend across a period however;
Metal group example:
In group 1 (alkali metals), the melting/boiling pts decrease as the atomic number increases. This is because there is an decrease in the attraction between the particles. (refer to bonding)
Non-metal group example:
In group 7 (halogens) the melting/boiling pts increase as the atomic number increases. This is because there is an increase in the attraction between the particles. (refer to bonding)
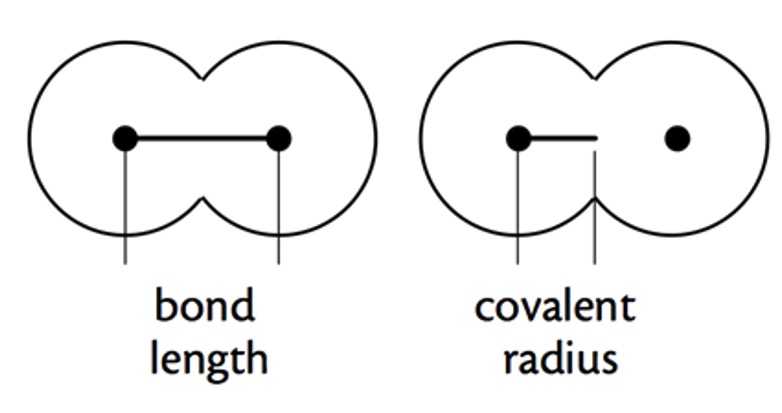
The covalent radius of an element is used to give an indication of its atomic size.
Covalent radius is half the distance between the nuclei of two covalently bonded atoms.
 Covalent radii of selected elements can be found in the data booklet on page 7.
Covalent radii of selected elements can be found in the data booklet on page 7.
Across a period (left to right) - Atomic Size decreases
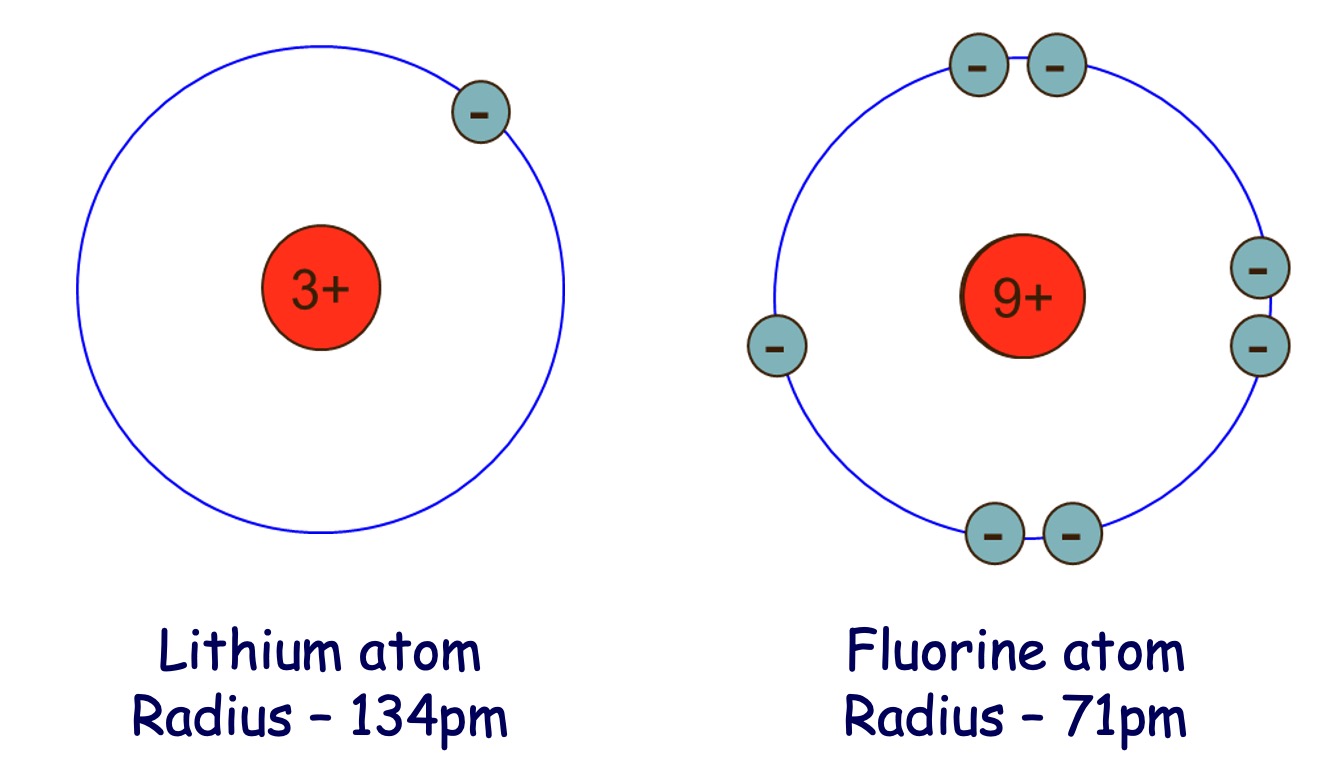
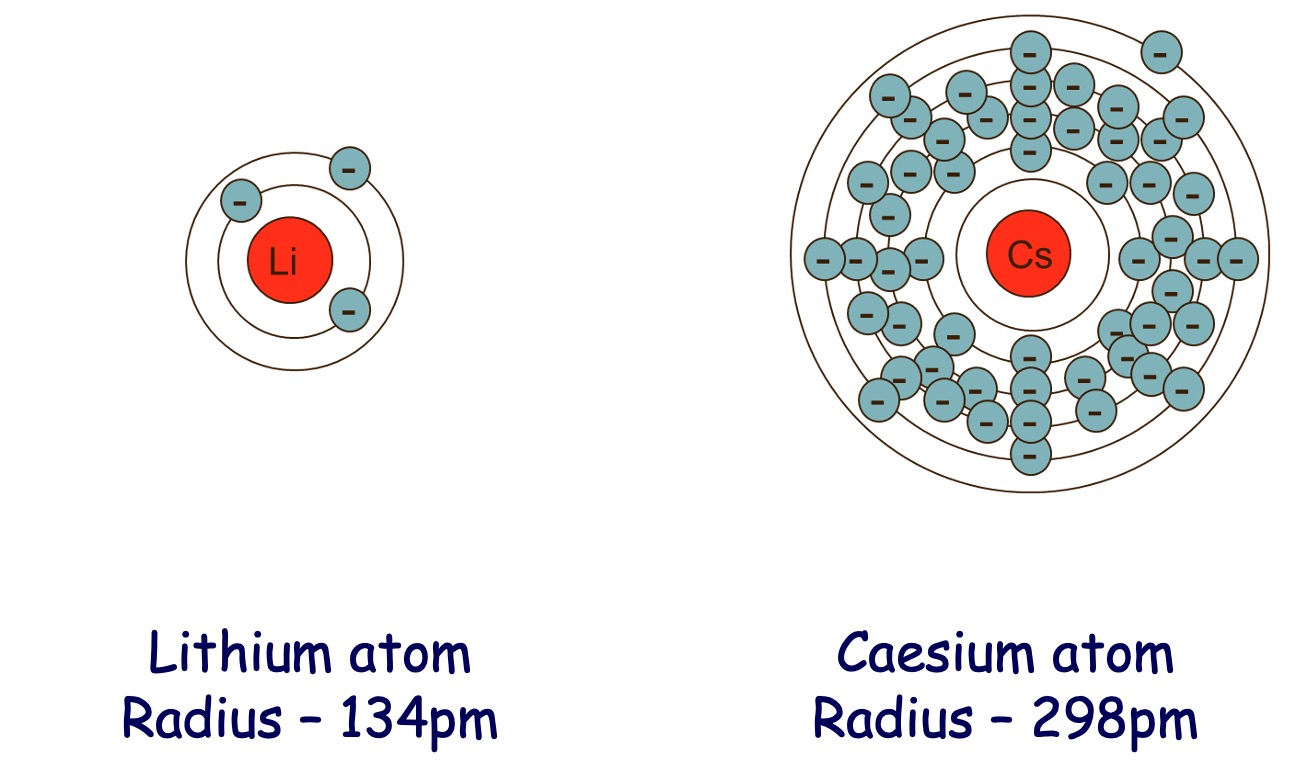
Down a group - Atomic Size increases.
The elements lithium to neon make up the second period of the Periodic Table. Identify the correct trend and explanation for the change in atomic size from lithium to fluorine.
The covalent radii of elements are listed on page 7 of the data book. Suggest why there are no values provided for the Noble Gases (Group 0).
The ionisation energy is the energy required to remove one electron from each atom in one mole of gaseous atoms of the element.
More than one electron can be removed from an atom so we can also have first, second, third and fourth ionisation energies.
A list of ionisation energies can be found in the data booklet on page 11.
The following equation shows the first ionisation for an element, E:
E (g) → E+(g) + e-
When writing equations to show ionisation you must show state symbols. The element must be in the gaseous state.
Metals and non-metals both undergo ionisation to form positively charged ions.
E.g.
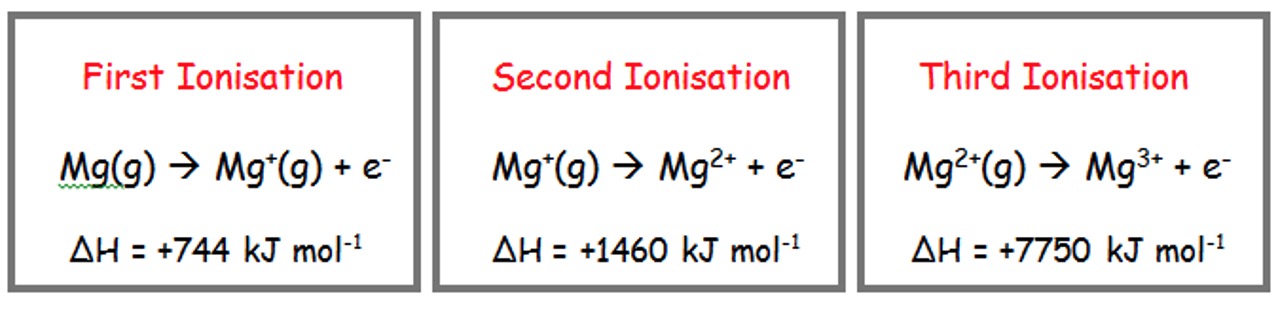
The total energy required to remove the three electrons above is:
744 + 1460 + 7750 = 9954 kJ mol-1.
Why is there such a large difference in ΔH between the 2nd and 3rd ionisation energy for Mg?
Across a Period (left to right) - Ionisation Energy increases.
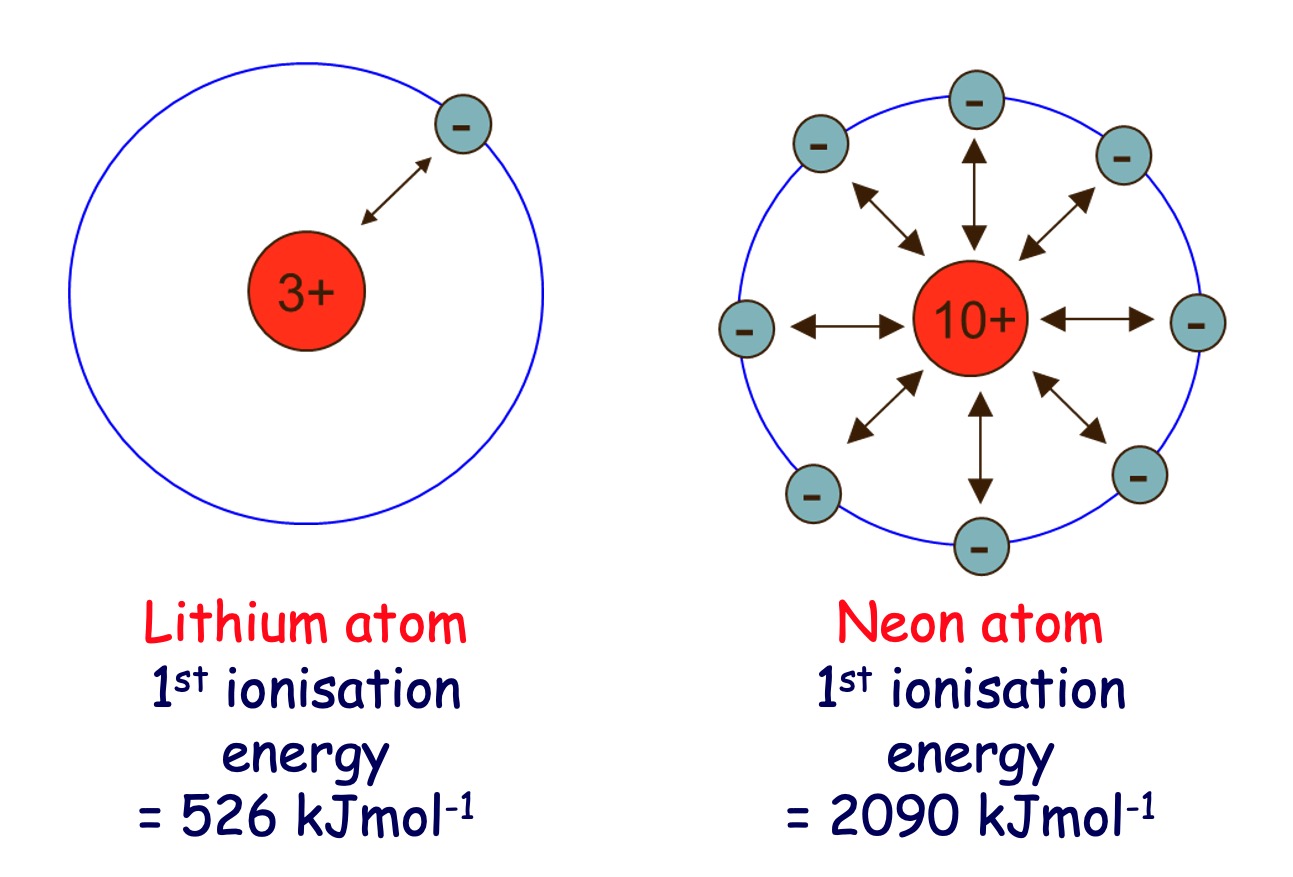
More protons are being added to the nuclei of the atoms. This results in an increase in nuclear charge.
The electrons in the outer energy level are attracted more strongly to the nucleus and therefore will be held more tightly, and more difficult to remove.
Down a Group - Ionisation energy decreases.
As you move down a group there are more energy levels.
Electron removed from the outer energy level is increasingly distant from the nucleus as a result of the increasing atomic size.
Electrons in the inner energy levels shield the outer electrons from the nucleus / nuclear charge.
Therefore they are not attracted as strongly resulting in less energy required to remove electron.
On crossing the third period from left to right there is a general increase in the first ionisation energy of the elements. Identify the correct explanation for increase in first ionisation energy increase across the period.
The spike graph shows the variation in successive ionisation energies of an element, Z. Identify the group of the Periodic Table which would contain element Z.
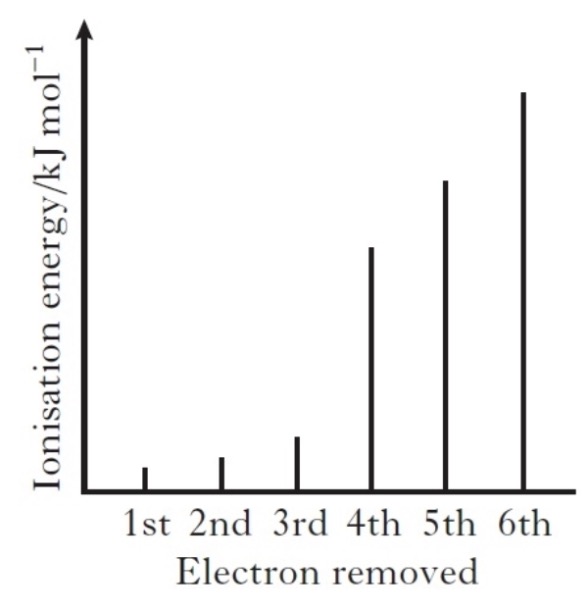
Identify which of the following elements would require the most energy to convert one mole of gaseous atoms into gaseous ions each carrying two positive charges.
Electronegativity is a measure of the attraction an atom involved in a bond has for the electrons of the bond.
Electronegativity values are based on the Pauling Scale, devised by Linus Pauling.
Values on the Pauling Scale range from 0 to 4.
A list of these values can be found in the data booklet on page 11.
The higher the number on the Pauling scale is, the greater the attraction an atom has for the bonding electrons.
Electronegativity values can be useful in predicting which type of bonding is most likely between two elements.
There are no electronegativity values for the monatomic gases. This is due to their electrical stability.

Fluorine is the most electronegative atom in the periodic table. It has a value of 4.
Across a period (left to right) - Electronegativity increases.
This is caused by an increase in nuclear charge / more protons as you move across a period from left to right.
Therefore it has a greater attraction for the bonding electrons.
Down a Group - Ionisation energy decreases.
This is caused by the additions of another energy level of electrons as you go down a group.
The inner energy levels shield the bonded electrons from the nucleus; therefore they are not as strongly attracted.
On descending Group 1 from lithium to caesium, the electronegativity of the elements decreases. Identify the correct explanation for the decrease in electronegativity.
The electronegativities of elements are listed on page 11 of the data book. Suggest why there are no electronegativity values provided for the Noble Gases (Group 0).
Atoms of nitrogen and element X form a bond in which the electrons are shared equally. Element X could be