The standard enthalpy of formation ΔHof is the enthalpy change when one mole of a substance is formed from its elements in their standard states.
The standard state of a substance, which is denoted by the small o, is its most stable state at a pressure of 1 atmosphere and at a specific temperature, usually taken as 298K.
The standard enthalpy of a reaction can be calculated from the standard enthalpies of formation of the reactants and products.
ΔHoreaction = ΣΔHof (products) - ΣΔHof (reactants)
Where,
ΔHoreaction = Standard change in enthalpy of reaction
ΣΔHof (products) = standard change in enthalpy of products
ΣΔHof (reactants) = standard change in enthalpy of reactants
The energy required to convert one mole of a solid element into separate atoms in the gas state. The most common one we use is carbon.
C(s) → C(g) ΔH = 716 kJ mol-1 page 11 of data book
The entropy (S) of a system is a measure of the degree of disorder of the system.
The greater the degree of disorder, the greater the entropy.
Solids have low disorder and gases have high disorder - Entropy increases as temperature increases.
For example, ice has a low degree of disorder compared to steam and therefore a low value for entropy.
There is a rapid increase in entropy at the melting point of a substance and an even more rapid and larger change in entropy at the boiling point. Entropy temperature graph.
The 3rd Law of thermodynamics - the entropy of a perfect crystal at 0 K is zero.
This is because the particles in a solid at 0 K are perfectly ordered an no longer vibrating.
The standard entropy of a substance So, is the entropy of 1 mol of the substance at a pressure of 1 atm at room temperature (usually 298K).
The units of entropy are J K-1 mol-1
For increase in entropy ΔSo will have a positive value, whereas a decrease in entropy ΔSo will give a negative value.
1. Define entropy and state its symbol.
2. State the effect of temperature on entropy.
3. Describe the effect on changing states on entropy.
4. What might have an entropy value = 0?
5. What are the standard conditions for standard entropy values?
6. What are the units of entropy?
7. How can entropy values for products and reactants give an entropy change?
A chemical reaction is described as being spontaneous/feasible if it tends to occur in the forward direction. (ΔSo is positive)
Burning petrol will easily burn to form carbon dioxide and water but turning these products back into petrol is virtually impossible.
A nail will rust but will not 'unrust' without a large input of energy.
HOWEVER, spontaneous does not mean instant.
The 2nd Law of thermodynamics
The total entropy of a reaction system and its surroundings always increases for a spontaneous process.
Heat energy released by the reaction system into the surroundings increases the entropy of the surroundings.
Heat energy absorbed by the reaction system from the surroundings decreases the entropy of the surroundings.
The change in standard entropy for a reaction system can be calculated from the standard entropies of the reactants and products.
ΔSoreaction = ΣSoproducts - ΣSoreactants
The standard free energy change, ΔGo can also be used to determine if a reaction is feasible and is defined as
ΔGo = ΔHo - TΔSo
For a reaction to be feasible ΔGo must be negative.
The temperature at which a reaction may be feasible can be estimated by considering the range of values for T for which ΔGo is negative.
At equilibrium ΔG = 0
The standard free energy change, ΔGo for a reaction can be calculated from the standard free energies of formation of the reactants and products using the relationship:
ΔGoreaction = ΣGoproducts - ΣGoreactants
The standard free energy of formation of an element (in its most stable form) is 0.
1. State the second law of thermodynamics
2. What is the effect of increasing temperature on the entropy of the surroundings?
3. State the equation relating free energy to enthalpy and entropy.
4. What value for the change in free energy would show a spontaneous reaction was likely.
5. Describe the difference between ΔG and ΔGo.
6. At what value of Δ G is equilibrium reached?
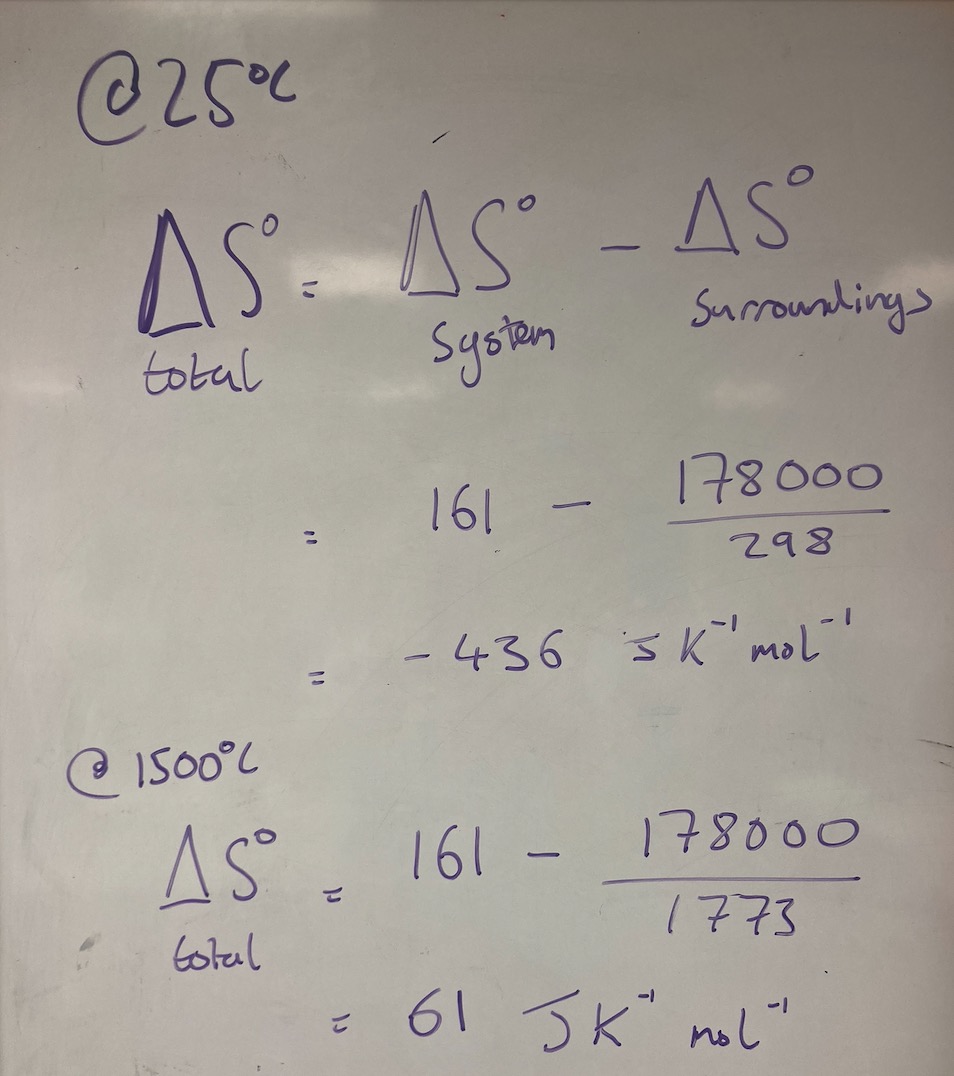
Calcium carbonate, present in limestone, is stable under normal atmospheric conditions. When it is in a volcanic area and it gets very hot, it can thermally decompose. Given the following information, use the Second Law of Thermodynamics to show why limestone is stable at 25oC, but not at 1500oC.
CaCO3(s) → CaO(s) + CO2(g)
ΔHo = +178 kJ mol-1
ΔSo = +161 J K-1 mol-1