Learning Intentions
- Describe the structure of an atom.
- Name and identify the three particles in the atom.
- Draw a diagram of an atom for elements with atomic number 1-20.
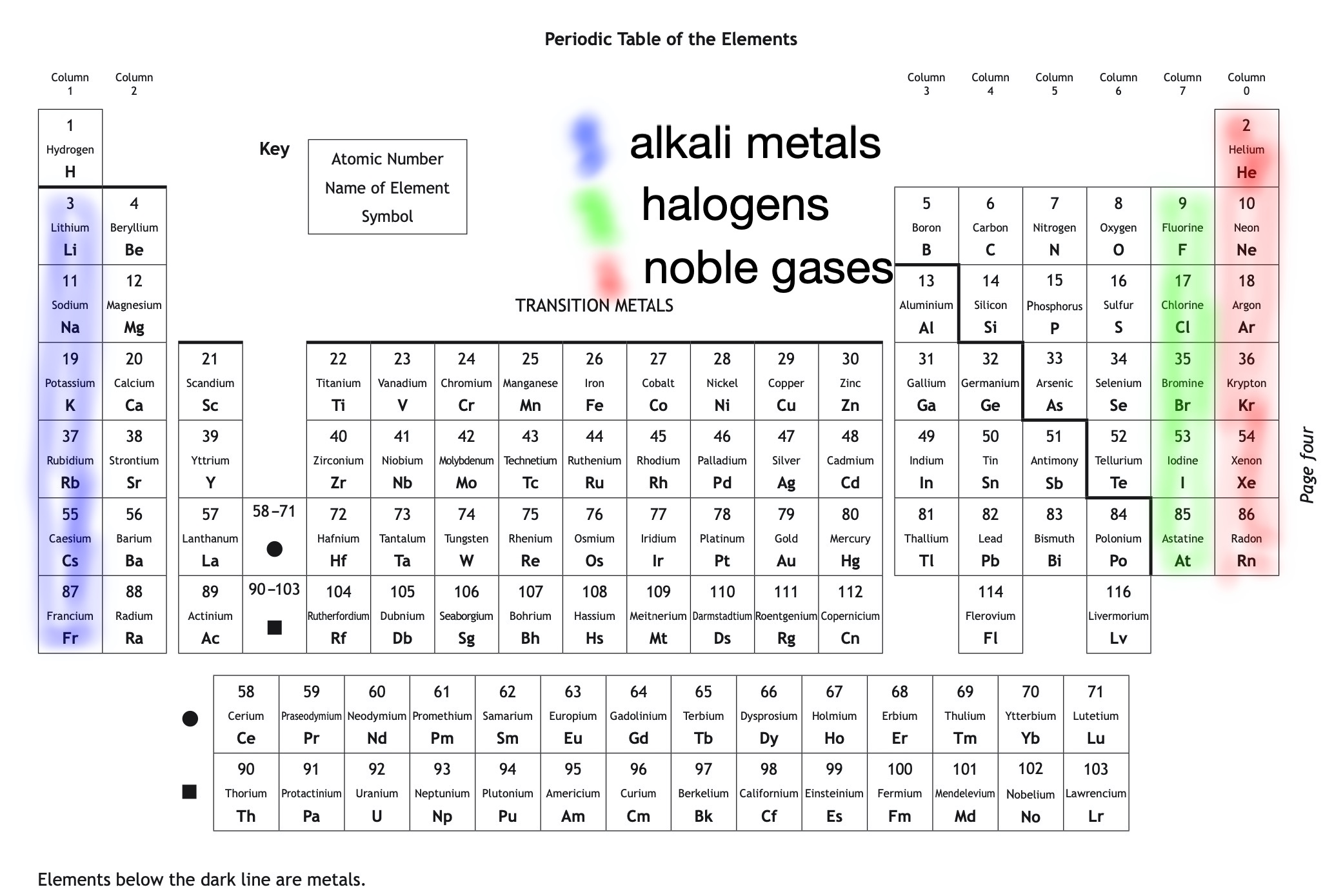
Elements - different types of atom
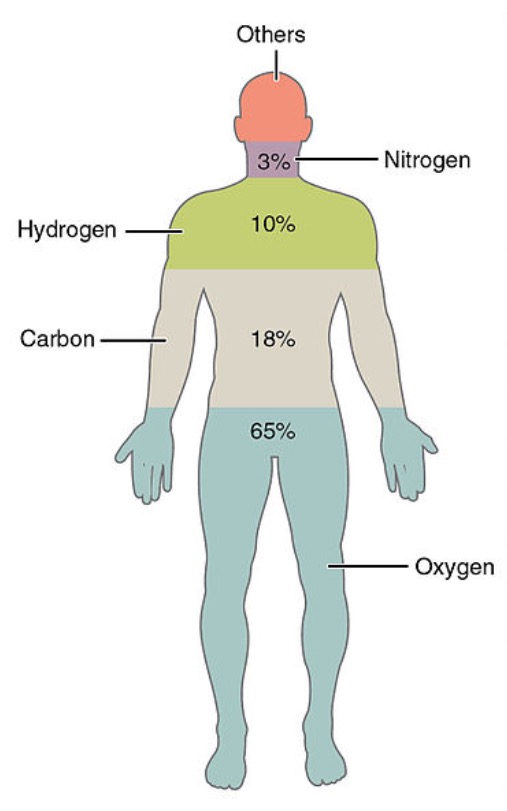
Elements are the simplest substances. There are about 100 different elements.
Each element is made up of very tiny particles called atoms, and each element is made up of just one particular type of atom, which is different to the atoms in any other element.
Gold is an element made up of only gold atoms.
Carbon is an element made up of only carbon atoms.
Atoms - the building blocks
John Dalton had the first ideas about the existence of atoms over 200 years ago.
However, it is only relatively recently that special microscopes (called electron microscopes) have been invented that can actually 'see' atoms.
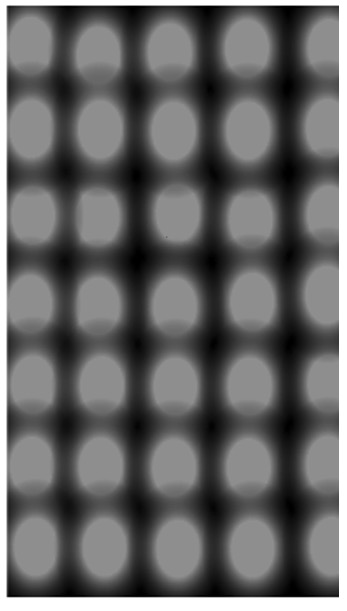
Electron microscopes produce images similar to this one. What could it be showing?
The grey blobs are individual lead atoms.
How small is an atom?

Atoms are very small - they are about 0.00000001 cm wide.
Think about the thickness of a crisp.
The number of atoms you would need to stack up to make the thickness of a crisp, is approximately the same number of crisps you would need to stack up to make the height of Mount Everest!
That's roughly 7 million crisps!
Sub atomic particles
For some time, people thought that atoms were the smallest particles and could not be broken into anything smaller.
Scientists now know that atoms are actually made from even smaller particles known as sub atomic particles. There are three types:

Notes - Atomic Structure
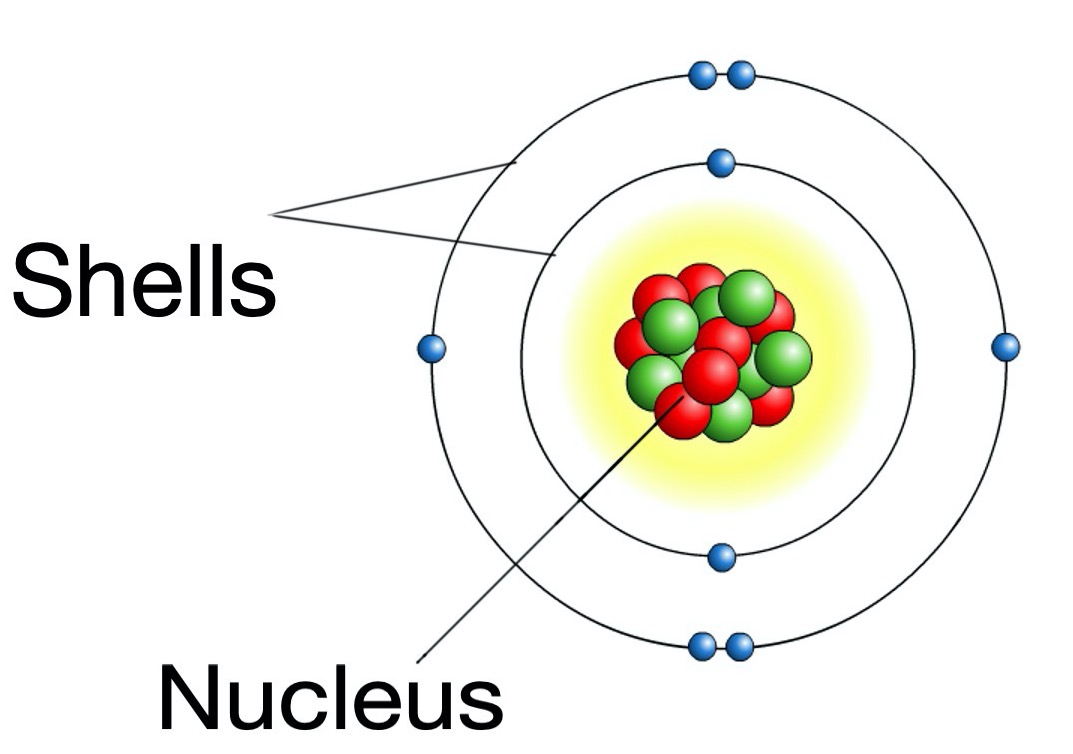
Protons, neutrons and electrons are not evenly distributed in an atom.
The protons and neutrons exist in a dense core at the centre of the atom. This is called the nucleus.
The electrons are spread out around the edge of the atom. They orbit the nucleus in layers called shells.
Notes - Properties of Sub Atomic Particles
|
Charge |
Mass (a.m.u.) |
Location |
| Proton |
+1 |
1 |
nucleus |
| Electron |
-1 |
negligible / 0 |
shell / orbital |
| Neutron |
0 |
1 |
nucleus |
An atom is neutral because the number of protons (+ve) = number of electrons (-ve)
Atomic number gives the number of electrons in an atom (and therefore number of protons in an atom, but not in an ion).
Mass number = protons + neutrons in an atom
Activity - Atomic Structure Posters
Use the steps below and the atom builder to make an atomic structure poster for one of the first 20 elements.
- Choose an element with an atomic number between 1 and 20.
- Using the atom builder, add the same number of protons to the nucleus as your chosen atomic number. For example, if you have chosen atomic number 12, add 12 protons.
- Add enough electrons to balance the charge of the protons.
- Click on the stable/unstable button.
- Add enough neutrons to make your atom stable.
- Using your diagram, make a poster showing the atomic structure of your atom. Your poster should have a title and include the following labels: proton, neutron, electron, nucleus and orbitals/shells.