


A chemical reaction can only occur if there is a successful collision between reactant molecules.
We can speed up a chemical reaction by:
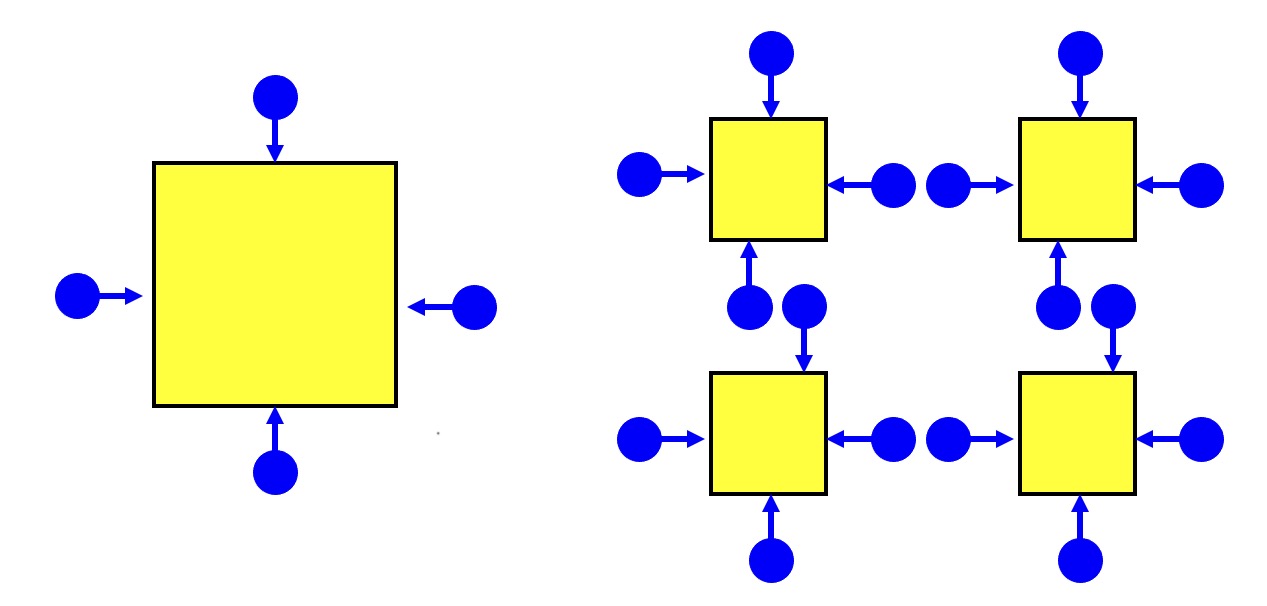
The smaller the particle size, the higher the surface area.
The higher the surface area, the greater the number of collisions that can occur at any one time.
The greater the number of collisions, the faster the reaction.
Therefore the smaller the particle size, the faster the reaction rate.
In this experiment you will investigate the relationship between particle size and rate of reaction.
Watch your teacher demonstrate how to carry out this experiment safely.
Record your data in a table like the one below.
| Time (s) | small chips | medium chips | large chips |
| Volume (cm3) | Volume (cm3) | Volume (cm3) | |
| 0 | |||
| 15 | |||
| 30 | |||
| 45 | |||
| 60 | |||
| 75 | |||
| 90 | |||
| 105 | |||
| 120 |
Plot a graph of your results. Your graph should be similar to the one in the section on interpreting graphs.
Write a conclusion that links what you have found out about the relationship between particle size and rate of reaction.
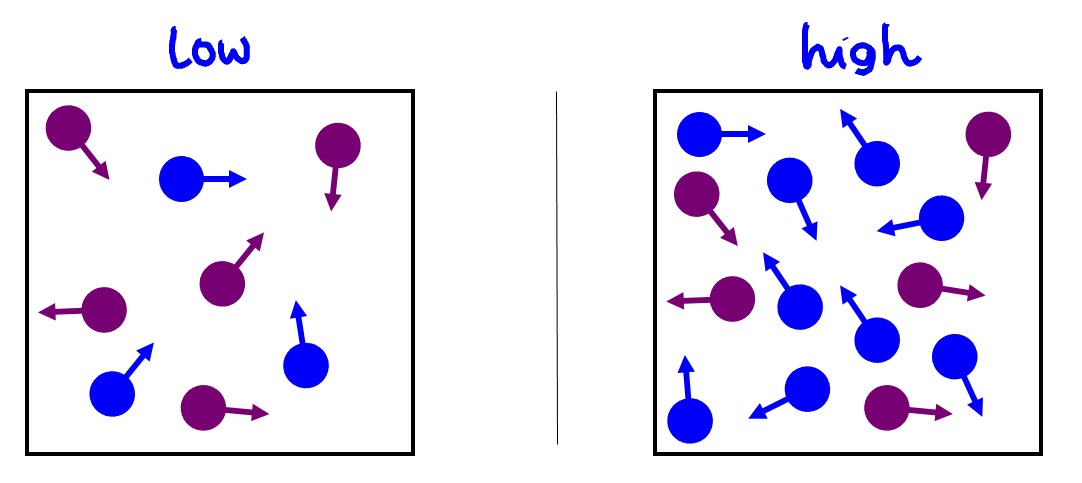
The higher the concentration, the higher the number of particles.
The higher the number of particles, the greater the chance of collisions that can occur.
The greater the number of collisions, the faster the reaction.
Therefore the higher the concentration, the faster the reaction rate.
In this experiment you will investigate the relationship between concentration and rate of reaction.
Watch your teacher demonstrate how to carry out this experiment safely.
Record your data in a table like the one below.
| Time (s) | small chips | medium chips | large chips |
| Volume (cm3) | Volume (cm3) | Volume (cm3) | |
| 0 | |||
| 15 | |||
| 30 | |||
| 45 | |||
| 60 | |||
| 75 | |||
| 90 | |||
| 105 | |||
| 120 |
Plot a graph of your results. Your graph should be similar to the one in the section on interpreting graphs.
Write a conclusion that links what you have found out about the relationship between concentration and rate of reaction.
The higher the temperature, the higher the energy the particles have.
The higher the energy, the faster the particles move.
The faster the particles move, the greater the chance that they can collide
The greater the number of collisions, the faster the reaction.
Therefore the higher the temperature, the faster the reaction rate.
A catalyst speeds up a chemical reaction and this is recovered chemically unchanged at the end of the reaction.
Enzymes are biological catalysts. They catalyse chemical reaction in living things.
In a reaction where a gas is produced, the gas can be collected and measured at regular time intervals throughout the reaction.
Two methods that are useful for collecting gasses when following a reaction are:
A - Collecting gasses over water
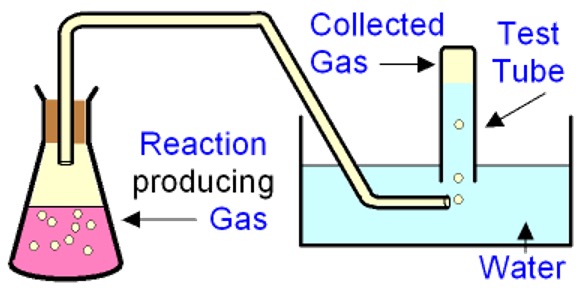
This can be used when relatively insoluble gases, or where a dry sample of gas is not required.
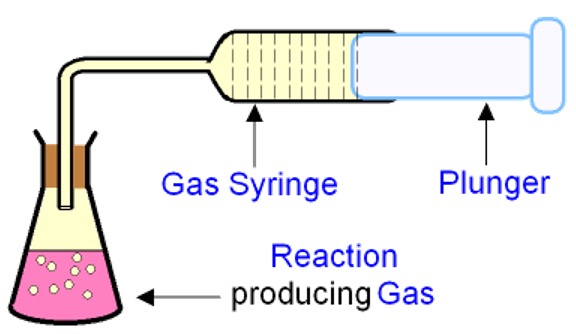
B - Gas Syringe
Graphs produced from following the volume of gas released during a reaction look similar to the one below.
The following information can be determined or predicted from the graph above:
During the course of a chemical reaction, reactants are being converted into products.
Measurement of the rate of reaction involves measuring the 'change in the amount' of a reactant or product in a certain time.
The rate of reaction changes as it progresses, being relatively fast at the start and slowing towards the end.
What is being measured is the average rate over the time interval chosen.
Reactions can be followed by measuring changes in concentration, mass and volume.
The average rate of reaction can be calculated using the formula below:
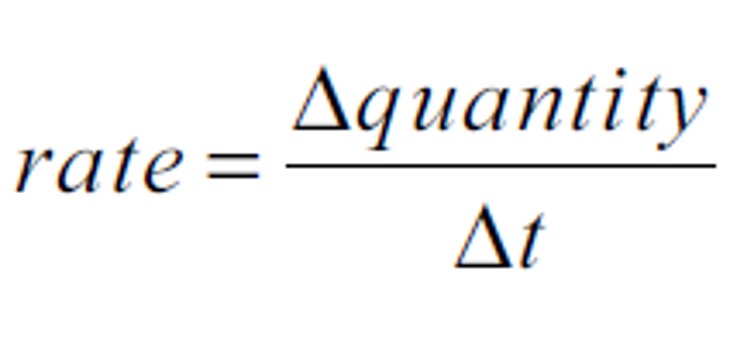
The unit of quantity is followed by the unit of time and a "-1".
e.g.
20cm3 of gas was produced in 10 minutes. Unit = cm3 min-1
or
5g of a solid was produced in 30 seconds. Unit = gs-1
or
0.5 moll-1 was reduced in 2 hours. Unit = moll-1 hr-1