

The rate of a chemical reaction is a measure of how fast the reaction takes place.
There are many variables that can affect the rate of a reaction.
The table below contains information about how changing concentration, temperature and particle size can affect the rate of reaction.
| Variable | Change to Variable | Change to Rate |
|---|---|---|
| Concentration | increase | speeds up reaction |
| decrease | slows down reaction | |
| Temperature | Increase | speeds up reaction |
| decrease | slows down reaction | |
| Particle Size | Increase | slows down reaction |
| Decrease | speeds up reaction |
Catalysts
A catalyst is a substance which speeds up a reaction.
A catalyst remains chemically unchanged at the end of a reaction and can be reused. The mass of the catalyst at the start of the reaction is the same at the end of the reaction.
Reactions that produce a gas can be used to monitor the rate of a reaction. The gas produced can be collected using a measuring cylinder or syringe. The volume of gas produced at set time intervals can be recorded.
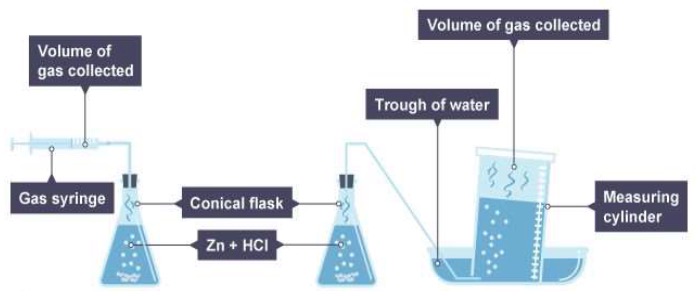
The change in mass of the reactants can be monitored by carrying out the reaction on a balance. The mass at set time intervals can be recorded.

We can use recorded data from an experiment to create a reaction rate graph. The reaction rate is related to the gradient (slope) of the line. The reaction has stopped when the line is horizontal.
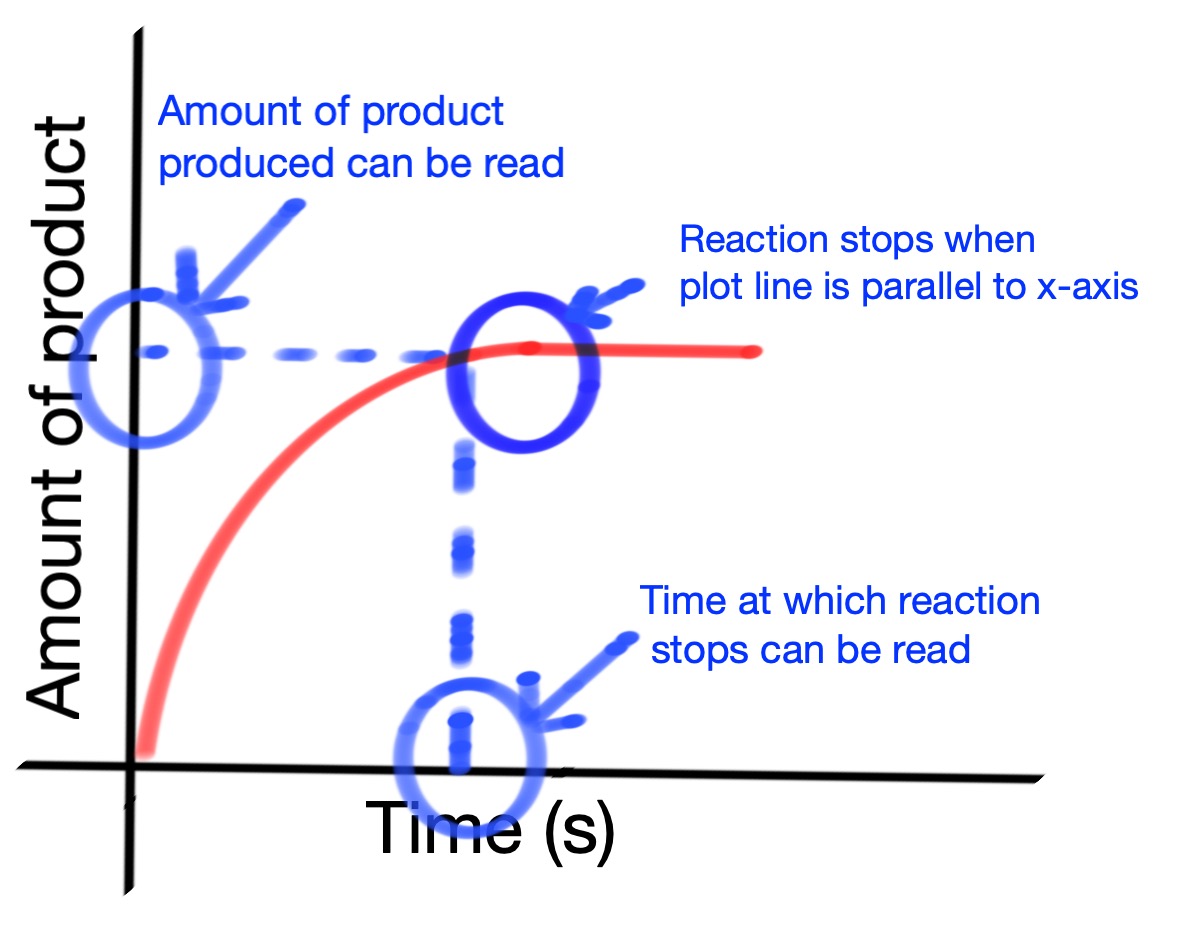
Can you answer the questions below based on the graph above?
1. When was the reaction the fastest?
2. When did the reaction stop?
3. How can the total amount of product from the reaction be found?
To compare reactions, multiple experiments can be plotted on the same graph.
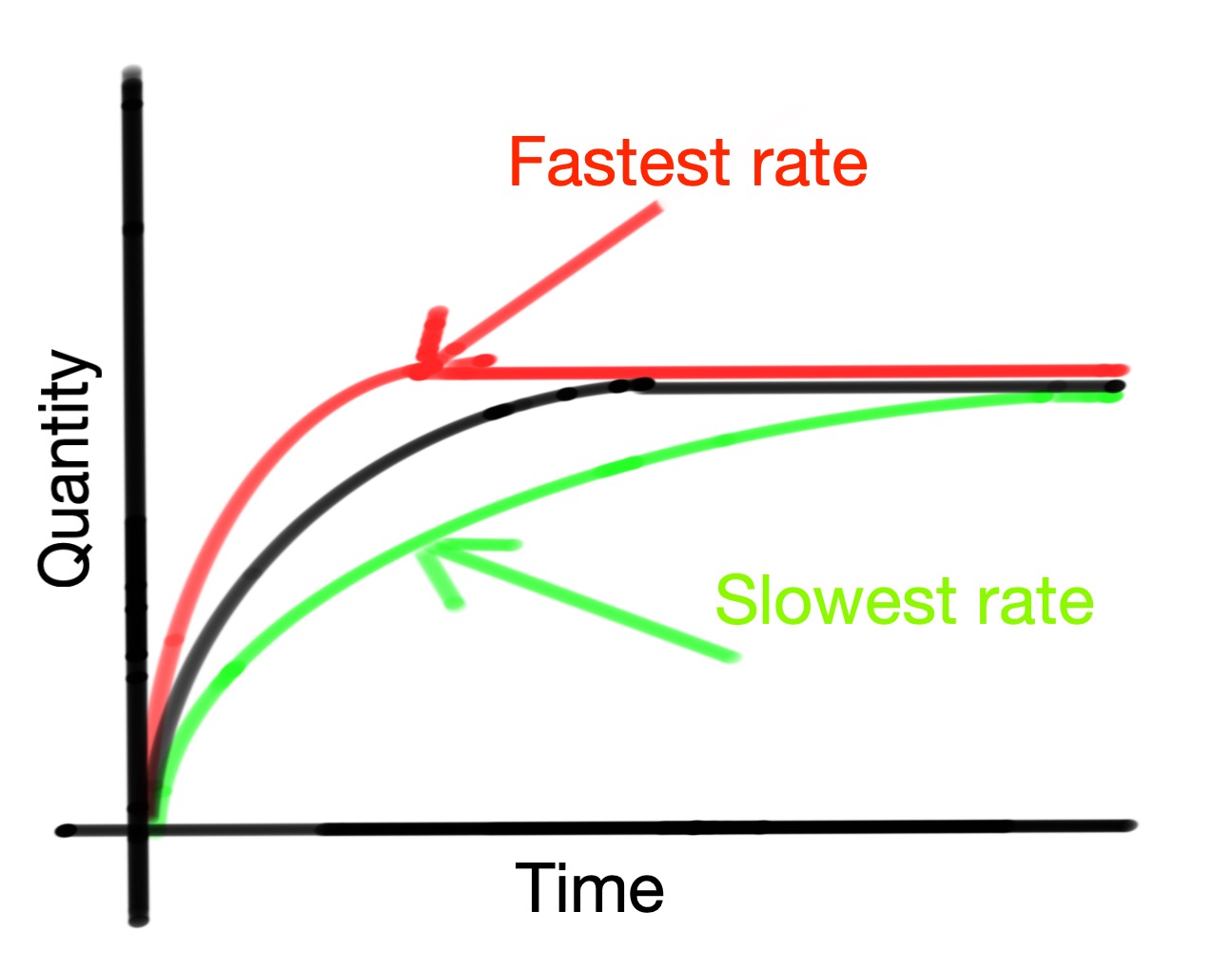
Use the graph above to help you answer the following questions.
1. Compare the black and red lines. What could cause an increase in reaction rate?
2. Compare the black and green lines. What could cause a decrease in reaction rate?
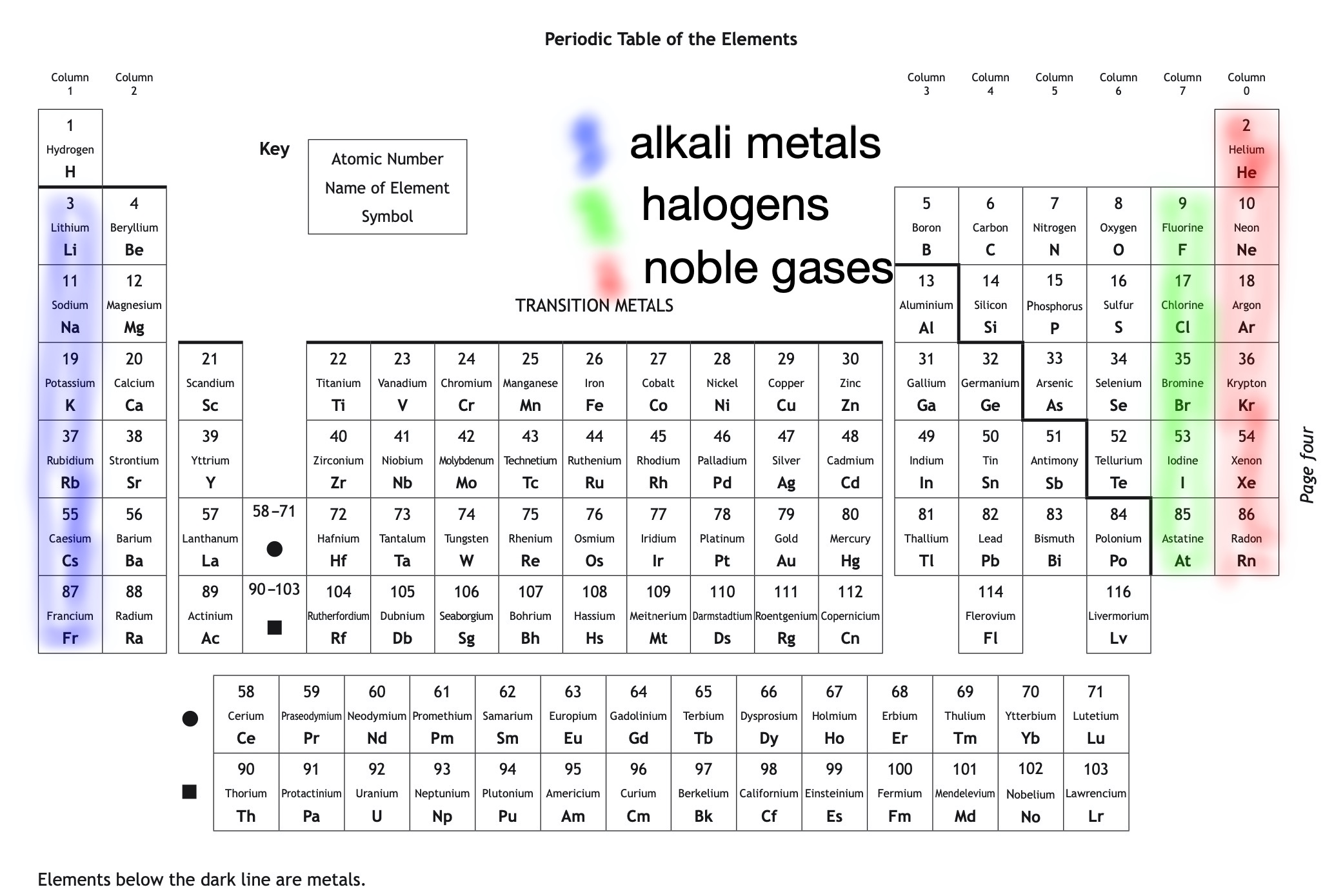
There are 118 known elements. These can be found in the Periodic Table. An element is a substance which contains only one type of atom. Each element has a different symbol and atomic number.
The Periodic Table is arranged in horizontal rows called periods and vertical columns called groups. The elements are arranged by atomic number and based on their chemical properties.
Elements in the same group of the Periodic Table have similar chemical properties.
Names of some of the groups in the periodic table can be seen below.
The diagram below shows the structure of the atom.
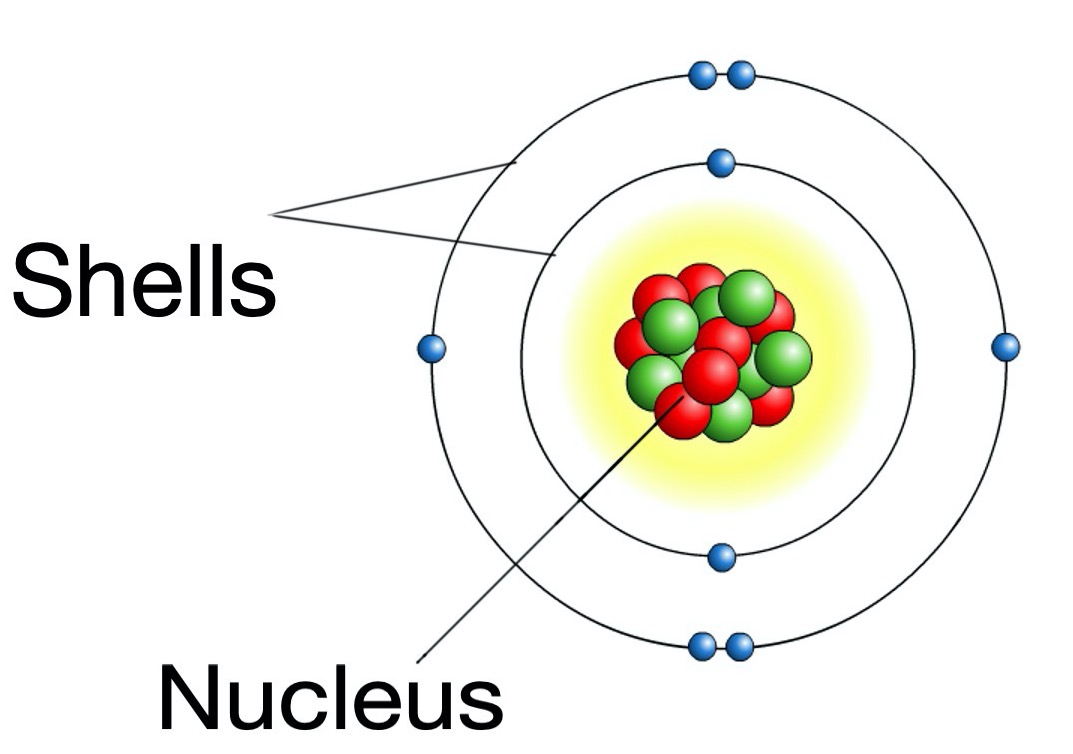
The atom is made up of a dense centre called the nucleus, which contains protons and neutrons.
The electrons are very light and are found in a space around the nucleus called the electron shell.
The table below shows the properties of the sub-atomic particles.
| Charge | Mass (a.m.u.) | Location | |
|---|---|---|---|
| Proton | +1 | 1 | nucleus |
| Electron | -1 | negligible / 0 | shell / orbital |
| Neutron | 0 | 1 | nucleus |
The number of protons, neutrons and electrons within an atom can be worked out by finding the atomic number and/or mass number of the atom.
Atoms are neutral because Number of protons = Number of electrons
Atomic Number = Number of protons
Number of Protons = Number of electrons
Mass Number = Number of protons + Number of neutrons
Number of Neutrons = Mass Number - Atomic Number
A compound is a substance that contains more than one type of atom. The chemical name of a compound can indicate the types of elements present. Name endings are used to indicate if there are two or three elements in a compound. These are shown in the table below.
| Name Ending | Information provided |
|---|---|
| IDE | 2 elements present in the compound |
| ATE/ITE | 3 elements in the compound, one is oxygen. |
Prefixes are used to indicate the number of atoms of a particular element present in a compound and these are shown below.
| Prefix | Number |
|---|---|
| mono | 1 |
| di | 2 |
| tri | 3 |
| tetra | 4 |
| penta | 5 |
| hexa | 6 |
The chemical formula of a compound indicates the elements present and the number of atoms of that element in the compound
e.g.
CO2
There is only one carbon atom and two oxygen atoms in this formula.
The chemical formula of a compound can be constructed by using one of three different methods:
The valency of an atom can be described as the number of bonds an atom can make. It can be determined using the valency of the main group element or the roman numeral given after the name of a transition metal.
The table below shows how the valency of the main groups in the periodic table.
| Group Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 0 |
|---|---|---|---|---|---|---|---|---|
| Valency | 1 | 2 | 3 | 4 | 3 | 2 | 1 | 0 |
The table below shows the valency that the roman numerals present in the compound name indicate.
| Roman Numeral | I | II | III | IV | V | VI | VII |
|---|---|---|---|---|---|---|---|
| Valency | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
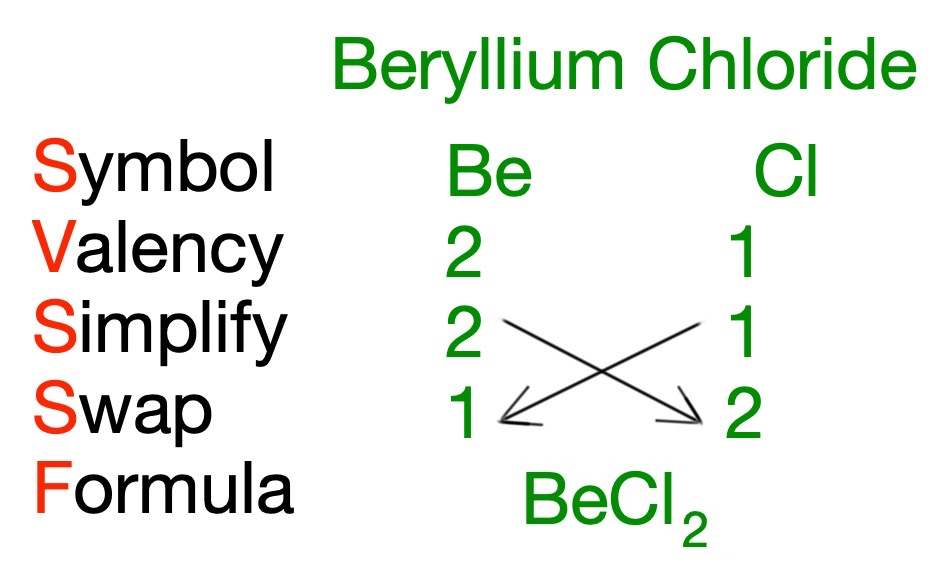
The chemical formula of a compound can be constructed using the following method.
| Symbol | The symbol of the elements is found form the periodic table. |
| Valency | The group or Roman Numerals indicate the valency of the elements. |
| Simplify | The valency numbers can be simplified if they can both be divided by another number other than 1. This is usually 2 or 3. |
| Swap | The valency numbers are swapped. |
| Formula | The formula of the compound is written out |
Example:
What is the chemical formula of beryllium chloride?
The gram formula mass (GFM) of a compound is the sum of all the relative atomic masses of each of the elements in the chemical formula.
Example:
What is the gram formula mass of beryllium chloride?
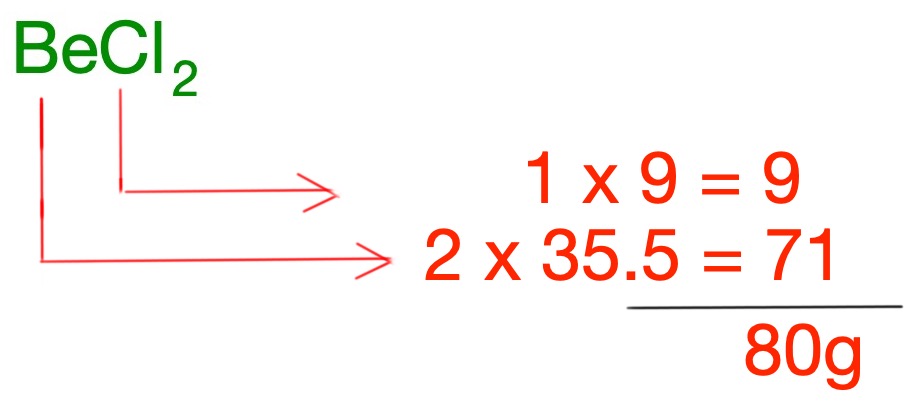
There are two main types of bonding, ionic and covalent.
Chemical reactions can be exothermic or endothermic
Chemical equations are used to describe a reaction including the reactants and the products
Reactants → Products
In a chemical equation the reactants and products are represented by their symbols/chemical formula.
The state of the compound/element must be included in a chemical equation
Copy the table below into your notes.
| Reactants | Start Temp (oC) | End Temp (oC) | Endo OR Exo |
|---|---|---|---|
| Sodium hydroxide + Hydrochloric acid | |||
| Sodium hydrogencarbonate + citric acid | |||
| Copper (II) Sulphate + Magnesium powder | |||
| Sulphuric acid Magnesium ribbon |
Follow link for instructions: https://edu.rsc.org/experiments/exothermic-or-endothermic-classifying-reactions/406.article
The pH scale is a measure of how acidic or alkaline a solution is.
Universal indicator can be used to indicate if a solution is acidic or alkaline
Everyday and laboratory substances can be acidic, neutral or alkaline
Making Acids and Alkalis
When a base is added to an acid, neutralisation will take place
When a neutralisation takes place using a metal oxide/hydroxide a salt and water are formed
Acid + Base → Salt + Water
There are two simple steps to follow when naming the salt formed as a result of a neutralisation
| Name of Acid | Second Part of Salt Name |
|---|---|
| Hydrochloric Acid | Chloride |
| Nitric Acid | Nitrate |
| Sulfuric Acid | Sulfate |
Carbon dioxide, sulfur dioxide and oxides of nitrogen are produced as a result of our continued use of fossil fuels
Acids are commonly present in food and drink as preservatives.