

Do pretest
Watch the video below. It should help you to decide the differenc between a chemicla an a physical change.

A change in which a new substance is made is called a "chemical change".
e.g. When you burn methane gas, the methane and oxygen change into new substances: carbon dioxide and water.
A physical change does not make anything new but may involve the original substance changing state e.g. from a solid to a liquid.
Melting, boiling, evaporating and condensing are all examples of physical changes.
Decide if the following are physical chamges or chemical reactions.
1. Melting ice is a...

2. Dissolving sugar in tea is a...

3. Making toast is a...

4. Water boiling is a...

5. A candle burning is a...
6. Water evaporating is a...

7. Burning wood in a fire is a...

8. A turkey cooking is a...
9. Making ice in a freezer is a...
10. Making steel from iron ore is a...

The video below shows lots of examples of chemical reactions. Note the differences between the reactants and products.
Do the five experiments and fill in the worksheet.
Watch the three videos below of chemical reactions.
Burning Sulfur
Exploding Ballon
Burning Methane
Collect the following equipment:
Instructions

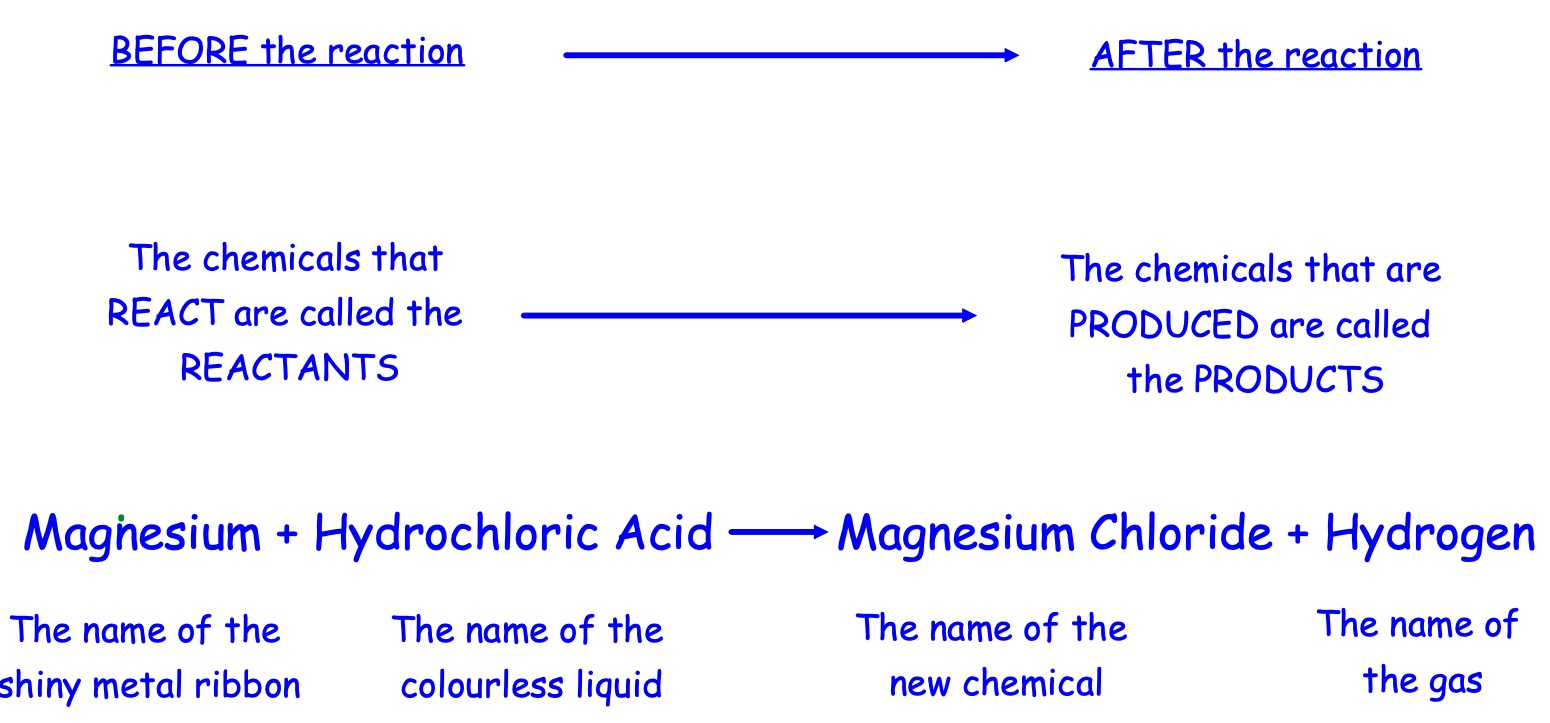
Work through the handout and write down the word equations.

Complete the word equtions for the chemical reactions below.
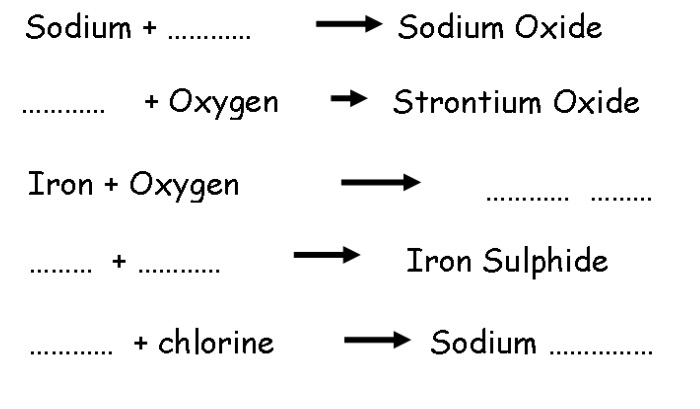

Write the word equations for the reactions below.
A piece of magnesium ribbon is added to hydrochloric acid. Magnesium chloride and hydrogen are produced.
The hydrogen gas is collected from 1 experiment 1. It is burned in oxygen. This produces water.
Complete the forms quiz below.


The diagram below shows which elements are represented by each colour.

Use the bonds to try and make the molecules in the table.
You can use the short bonds or the bendy ones.
All the holes on each atom must be filled
| Name | Elements | Formula | Picture |
|---|---|---|---|
| Oxygen | O2 |

|
|
| Water | hydrogen and oxygen |

|
|
| Carbon Dioxide | CO2 |

|
|
| Methane | carbon and hydrogen |

|
|
| Hydrogen Chloride | HCl |

|
Click on the simulation below to find out how adding solute affects the concentration.
Increasing concentration means dissolving more particles into a solution.
Diluting means adding more water.
For any chemical reaction to happen particles must collide with each other.
The more people in a room the more chance you will bump into someone. It's exactly the same in a chemical reaction.
Increasing the concentration makes reactions go faster because there are more collisions.
For any chemical reaction to happen particles must collide with each other.
The only part of a particle that can be hit is the outside The amount of a substance that can be hit is called the "surface area"
If you break up a large lump into smaller pieces you increase the surface area allowing more collisions to happen.
This is why everything will react faster if you break it into smaller pieces.
| Temperature (oC) | Time for tablet to disapear (s) |
|---|---|
Increasing temperature has 2 effects.
It speeds up the particles and so increases the number of collisions that occur.
More importantly, for particles to react they must collide with enough force or they just bounce off each other. Because the particles are moving faster there are far more successful collisions.
For any chemical reaction to happen particles must collide with each other.
Catalysts work by holding one of the particles steady. It is therefore more likely other particles will collide increasing the number of reactions.
Catalysts speed up the rate of a chemical reaction. Catalysts are not used up. Catalysts can be biological, called enzymes.

Electrolysis heppens when electricity is used to break up a compound into its separate elements.

Collect the followig equipment:
Set it up as in the diagram below.
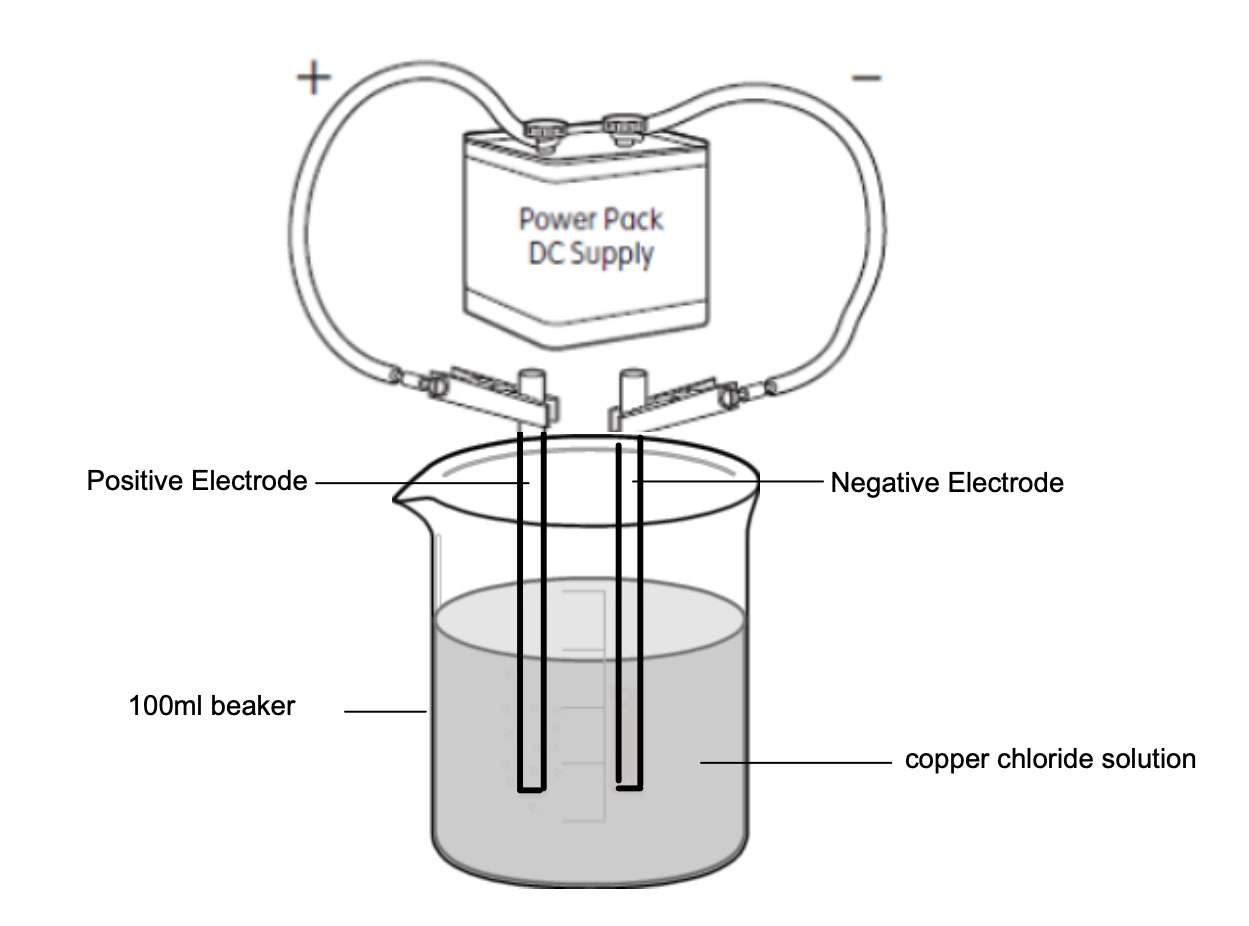

What do you see?
What do you smell?

Watch the video below on electrolysis. Answer the questions that appear throughout the video.

Luigi Galvani (1737- 98) was an Italian trained in medicine and interested in physics.
His interest in anatomy and physics led him to look at the effect of electricity on the muscles of animals, particularly frogs.
The process of galvanising is named after him.

He sometimes used electricity from thunderstorms and attached the frog's legs to iron fences and other large metal objects.
He found that by connecting two different metals into the frog's legs, the muscle contractions occurred without any apparent source of electricity.
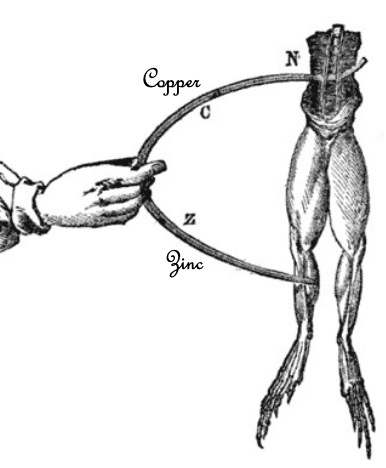
He thought the electricity was in the nerve juices in the muscles.

Another Italian, Alessandro Volta, realized that it was the two metals themselves which were generating the electricity to cause the nerve contractions in the muscles.
The term voltage and the units volts are named after him.
This is a similar experiment to the one Galvani did using some 'Nerve Juice' and two different metals (but not frog's legs).
Instructions
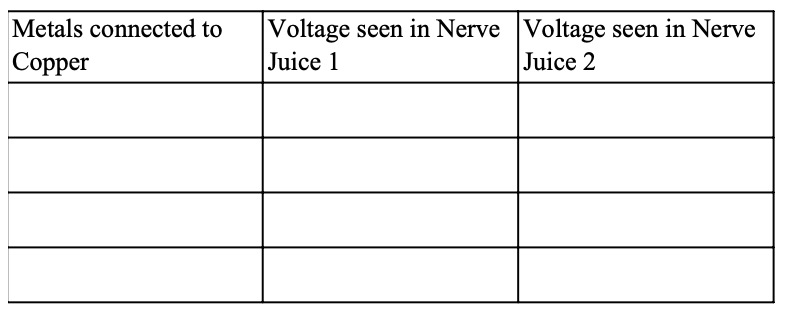

When two different metals are connected like this to produce electricity, we say we have made an electrochemical cell, sometimes just called a cell.
More than one cell connected together is called a battery.
The word battery is often used, incorrectly, instead of cell.
Challenge 1
Can you get one of your cells to power a buzzer?
Which cell will you choose?
Results
Metals chosen: _________ and ______
Solution chosen: ____________
Challenge 2
How many LEDs can you get to light up all together?
Will one cell make them all light up or will you need more?
Results
Draw a circuit diagram for your circuit.
Watch the video below on the hitory of the Electrochemical Cell
Grant observed the colour of trainers pupils were wearing in the Science corridor. Here are his results:
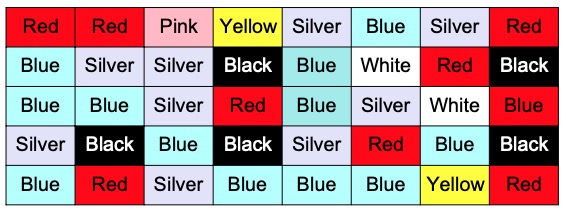
Using graph paper, display this data in a bar chart.
The table below shows how quickly a beaker of water was heated up using a Bunsen Burner.
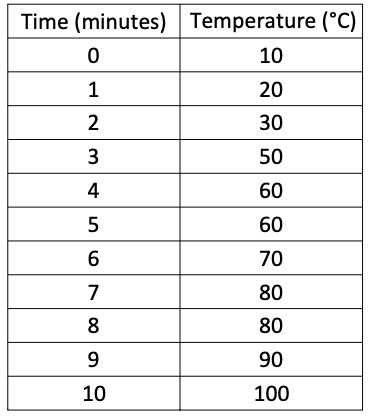
Using graph paper, plot the information as a line graph.